MAP around 80 mmHg
The MAP refers to the average pressure within the arterial system that is felt by
Normal MAP for adults organs in the body
A MAP greater than 60 mm Hg is needed to adequately perfuse and sustain the vital
organs of an average person under most conditions.
Around 5-10 cmH2O
Normal PEEP Positive End Expiratory Pressure
Pressure to keep alveolus open when expiring
pH: 7.35-7.45
PaO2: 80-100%
ABG values
PaCO2: 35-45 (acidic= 45, alkalotic= 35)
HCO3: 22-26
amount of air you inhale and exhale during a normal breath
Tidal volume
amount to program on ventilator for the amount of air to be pushed into the lungs
Apnea absence of breathing
periods of apnea alternating irregularly with a series of shallow breaths of equal
Ataxic breathing
depth
pattern of respiration characterized by alternating periods of apnea and deep, rapid
Cheyne strokes ventilation
breathing
rapid, deep breathing associated with dyspnea. They are the body's attempt to
Kussmaul breathing
reverse metabolic acidosis through the exhalation of excess CO2.
, 1. Buffer system-immediate (proteins, ions absorbing and releasing H)
2. Respiratory system -within minutes but not long term (changes in O2)
Normal regulation of acids and bases
3. Renal system - slowest (hours to days) but more sustainable(changes in H &
HCO3)
Hyperventilation-lower PaCO2-INCREASES pH
PaCO2=partial pressure in blood
Hyperventilation & hypoventilation effects
Hypoventilation-raises PaCO2-DECREASES pH
Metabolic Acidosis problem (decreased Triggers hyperventilation-increase rate and depth to decrease CO2 levels and
pH) triggers hyper/hypoventilation? INCREASE pH
Metabolic Alkalosis problem (increased Triggers hypoventilation-decreased rate and depth increases CO2 and DECREASES
pH) triggers hyper/hypoventilation? pH
When respiratory issues are occurring They reabsorb bicarb and increase excretion of hydrogen. Increased bicarb in blood
causing acidosis (low pH), what do the increases the pH back to normal
kidneys do to compensate?
When respiratory issues are occurring They excrete bicarb and retain hydrogen to increase urine alkalinity and decrease
causing alkalosis (high pH), what do the bicarb in the blood this lowering pH back to normal
kidneys do to compensate?
Decreased pH
What happens to pH, HCO3, respiratory Decreased bicarb
rate/depth and CO2 when it is an ACID Decreased rate/depth
Increased CO2
Increased pH
What happens to pH, HCO3, respiratory Increased bicarb
rate/depth and CO2 when it is an BASE Increased rate/depth
Decreased CO2
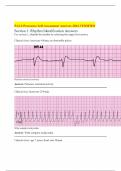
Review un/part/fully compensated pH notebook
disturbances
Causes & pathophysiology pH Page 288
disturbances
Clinical manifestations of Acidosis Page 289
Cardiac:
resp: v fib
meta: dysrhythmias
(due to compensation r/t high K)
Skin:
resp: warm, flushed
meta: cold, clammy
Gastro:
How are the clinical manifestations of resp: no signs
acidosis different for respiratory vs meta: N/V/D, pain
metabolic? Neuromuscular:
resp: seizures
meta: muscle
weakness
Respiratory:
resp: hypovolemia
with hypoxia
meta: deep, rapid
respirations
Manifestations of alkalosis chart page 289