OpenStax Organic Chemistry: A Tenth Edition Student Solutions Manual
Chapter 22 – Carbonyl Alpha-Substitution Reactions
Solutions to Problems
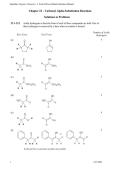
22.1–22.2 Acidic hydrogens in the keto form of each of these compounds are bold. One of
these hydrogens is removed by a base when an enolate is formed.
Number of Acidic
Hydrogens
(a) 4
(b) 3
(c) 3
(d) 2
(e) 4
(f) 5
In (d) and (f), cis and trans enolates are possible.
1 12/1/2023
, OpenStax Organic Chemistry: A Tenth Edition Student Solutions Manual
22.3
The first two monoenols are more stable because the enol double bond is conjugated with
the carbonyl group.
22.4
22.5 Alpha-bromination, followed by dehydration using pyridine, yields the enone below.
11/6/2023 2
, OpenStax Organic Chemistry: A Tenth Edition Student Solutions Manual
22.6
The mechanism of the ester-forming step is a nucleophilic acyl substitution, which was
described in Chapter 21.
22.7 Hydrogens α to one carbonyl group (or nitrile) are weakly acidic. Hydrogens α to two
carbonyl groups are much more acidic, but they are not as acidic as carboxylic acid
protons.
(a) (b) (c)
(d) (e) (f)
22.8 Nitriles are weakly acidic because the nitrile anion can be stabilized by resonance
involving the π bonds of the nitrile group.
3 11/6/2023
Chapter 22 – Carbonyl Alpha-Substitution Reactions
Solutions to Problems
22.1–22.2 Acidic hydrogens in the keto form of each of these compounds are bold. One of
these hydrogens is removed by a base when an enolate is formed.
Number of Acidic
Hydrogens
(a) 4
(b) 3
(c) 3
(d) 2
(e) 4
(f) 5
In (d) and (f), cis and trans enolates are possible.
1 12/1/2023
, OpenStax Organic Chemistry: A Tenth Edition Student Solutions Manual
22.3
The first two monoenols are more stable because the enol double bond is conjugated with
the carbonyl group.
22.4
22.5 Alpha-bromination, followed by dehydration using pyridine, yields the enone below.
11/6/2023 2
, OpenStax Organic Chemistry: A Tenth Edition Student Solutions Manual
22.6
The mechanism of the ester-forming step is a nucleophilic acyl substitution, which was
described in Chapter 21.
22.7 Hydrogens α to one carbonyl group (or nitrile) are weakly acidic. Hydrogens α to two
carbonyl groups are much more acidic, but they are not as acidic as carboxylic acid
protons.
(a) (b) (c)
(d) (e) (f)
22.8 Nitriles are weakly acidic because the nitrile anion can be stabilized by resonance
involving the π bonds of the nitrile group.
3 11/6/2023