1|Page
CEM 141 MSU Exam 1newest 2026
already graded A+
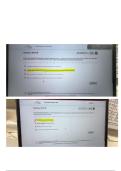
The statement "If I add cold milk to hot coffee the temperature will decrease," is a? - - ANS✔️--
Claim, always include an explanation and reasoning to the claim.
Scientific Model - - ANS✔️--Models are used to make testable predictions
How much is a picometer? - - ANS✔️--0.1 x 10^-12 meters
Atomic Force Microscopy/ Scanning Tunneling Microscopy - - ANS✔️--Microscopes that now
allows us to view atoms.
Leaves are green in the summer because? - - ANS✔️--A plant's leaves are green in the summer
because they contain chlorophyll. Chlorophyll is a natural pigment that absorbs all wavelengths
in the visible spectrum except green.
How many naturally occurring elements exist? - - ANS✔️--91
, 2|Page
What was Dalton's Atomic theory? (1808) - - ANS✔️--- Elements are composed of small
particles called Atoms.
- Atoms of a given element are different from atoms of other elements.
-Chemical reactions happen when atoms are rearranged.
- Compounds contain 2 or more elements.
What was J.J. Thomson's experiment, and what did he discover? - - ANS✔️--Using a Cathode
Ray Tube, he noticed that particles emerged to the cathode disk, and moved to another, the
anode. These particles could be deflected by electrical fields that would indicate that they would
be negatively charged. They were also deflected by magnetic fields, which showed the particles
carried the charge as the ray was bent. The most important observation was that the type of
cathode used did not matter, as these particles were still emitted. Therefore, Thomson deduced
that the particles were universal and were in fact negatively charged components of the atom.
(The Electron)
What was Ernest Rutherford's experiment, what did he discover? - - ANS✔️--Ernest Rutherford
fired Alpha particles (Hydrogen atoms) at a thin sheet of gold foil. Had Thomson's theory been
right about the plum pudding model, then the atoms should have all passed right through the
sheet with no exceptions. However, a small ratio of these particles were deflected. With this
information, Rutherford figured there was a small dense nucleus in the atom and that most of the
atom was empty space.
CEM 141 MSU Exam 1newest 2026
already graded A+
The statement "If I add cold milk to hot coffee the temperature will decrease," is a? - - ANS✔️--
Claim, always include an explanation and reasoning to the claim.
Scientific Model - - ANS✔️--Models are used to make testable predictions
How much is a picometer? - - ANS✔️--0.1 x 10^-12 meters
Atomic Force Microscopy/ Scanning Tunneling Microscopy - - ANS✔️--Microscopes that now
allows us to view atoms.
Leaves are green in the summer because? - - ANS✔️--A plant's leaves are green in the summer
because they contain chlorophyll. Chlorophyll is a natural pigment that absorbs all wavelengths
in the visible spectrum except green.
How many naturally occurring elements exist? - - ANS✔️--91
, 2|Page
What was Dalton's Atomic theory? (1808) - - ANS✔️--- Elements are composed of small
particles called Atoms.
- Atoms of a given element are different from atoms of other elements.
-Chemical reactions happen when atoms are rearranged.
- Compounds contain 2 or more elements.
What was J.J. Thomson's experiment, and what did he discover? - - ANS✔️--Using a Cathode
Ray Tube, he noticed that particles emerged to the cathode disk, and moved to another, the
anode. These particles could be deflected by electrical fields that would indicate that they would
be negatively charged. They were also deflected by magnetic fields, which showed the particles
carried the charge as the ray was bent. The most important observation was that the type of
cathode used did not matter, as these particles were still emitted. Therefore, Thomson deduced
that the particles were universal and were in fact negatively charged components of the atom.
(The Electron)
What was Ernest Rutherford's experiment, what did he discover? - - ANS✔️--Ernest Rutherford
fired Alpha particles (Hydrogen atoms) at a thin sheet of gold foil. Had Thomson's theory been
right about the plum pudding model, then the atoms should have all passed right through the
sheet with no exceptions. However, a small ratio of these particles were deflected. With this
information, Rutherford figured there was a small dense nucleus in the atom and that most of the
atom was empty space.