Tuesday, August 27, 2024 7:50 AM
Principals and particles Page 1
,Principals and particles Page 2
,Principals and particles Page 3
, 8/29
Wednesday, August 28, 2024 11:36 PM
Principals and particles Page 4
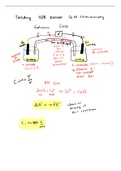
The first part of the course (“Principles and Particles”) introduces the foundations of chemistry by explaining the nature of matter at the atomic level. It covers atomic structure, subatomic particles, isotopes, and how experimental evidence led to modern atomic theory. Key physical principles such as energy, forces, and electrostatics are used to explain how particles interact. This section also introduces early quantum ideas, atomic orbitals, electron configurations, periodic trends, and how these properties determine chemical behavior. The emphasis is on connecting measurable macroscopic properties to microscopic particle models. The quantum mechanics unit builds the mathematical and conceptual framework needed to describe electrons accurately. It introduces wave–particle duality, the Schrödinger equation, quantized energy levels, and atomic orbitals as probability distributions rather than fixed paths. Topics include operators, wavefunctions, normalization, expectation values, and quantum numbers. These ideas are then applied to atoms and molecules to explain bonding, orbital shapes, and electronic structure. This section provides the theoretical basis for why atoms bond the way they do and why molecules have specific shapes and energies. The later units focus on molecules and spectroscopy. Molecular structure is explained through bonding models, molecular orbitals, hybridization, symmetry, and molecular geometry. Energetics, bonding interactions, and electron delocalization are emphasized. Spectroscopy (IR, NMR, and related techniques) is introduced as an experimental tool to probe molecular structure by observing how molecules interact with electromagnetic radiation. Students learn how vibrational, rotational, and nuclear spin transitions produce characteristic spectra that reveal functional groups and molecular environments. Together, these sections connect theory to real experimental data, showing how chemists determine structure and behavior at the molecular level.








Kwaliteit die je kunt vertrouwen: geschreven door studenten die slaagden en beoordeeld door anderen die dit document gebruikten.
Geen zorgen! Je kunt voor hetzelfde geld direct een ander document kiezen dat beter past bij wat je zoekt.
Geen abonnement, geen verplichtingen. Betaal zoals je gewend bent via Bancontact, iDeal of creditcard en download je PDF-document meteen.

“Gekocht, gedownload en geslaagd. Zo eenvoudig kan het zijn.”