Teas 7 Chemistry
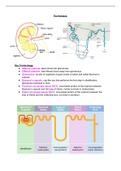
Solid to Liquid - Answer-Melting (absorbs energy)
Liquid to Solid - Answer-Freezing, (releases energy)
Liquid to Gas - Answer-vaporization/absorbs energy
Gast to Liquid - Answer-condensation/ release energy
Gas to Solid - Answer-deposition/ release energy
Solid to Gas - Answer-sublimation
Matter - Answer-Anything that takes up space
Mass - Answer-the amount of matter in an object
How Does Matter Change? - Answer-Matter usually changes state when you add or take away heat,
which changes the temperature of the matter
Substance - Answer-A single kind of matter that is pure and has a specific set of properties.
examples of substances - Answer-1. Water
2. Alcohol
, 3. Oil
4. Food coloring
Solids - Answer-The particles in solids are tightly packed
Liquids - Answer-Liquids are also a condensed phase, however, in contrast to solids, the particles have
some translational kinetic energy, which means they have freedom to move
gas phase - Answer-the phase of matter in which atoms or molecules can move essentially
independently of one another
Which state(s) of matter take the shape of their container? - Answer-Liquids and gases take the shape of
their containers since, they do not have definite shape and volume. Only solids have definite shape and
volume.
SOLIDS - Answer-have the least volume
LIQUIDS - Answer-have a definite volume
GASES - Answer-have maximum volume
Which of the following is correct?
(a) Solids have a definite shape and definite volume.
(b) Liquids have a definite volume but no definite shape.
(c) Gases have a definite volume but no definite shape.
(d) Liquids have both definite shape and definite volume. - Answer-(a) Solids have definite shape and
definite volume.
Solid to Liquid - Answer-Melting (absorbs energy)
Liquid to Solid - Answer-Freezing, (releases energy)
Liquid to Gas - Answer-vaporization/absorbs energy
Gast to Liquid - Answer-condensation/ release energy
Gas to Solid - Answer-deposition/ release energy
Solid to Gas - Answer-sublimation
Matter - Answer-Anything that takes up space
Mass - Answer-the amount of matter in an object
How Does Matter Change? - Answer-Matter usually changes state when you add or take away heat,
which changes the temperature of the matter
Substance - Answer-A single kind of matter that is pure and has a specific set of properties.
examples of substances - Answer-1. Water
2. Alcohol
, 3. Oil
4. Food coloring
Solids - Answer-The particles in solids are tightly packed
Liquids - Answer-Liquids are also a condensed phase, however, in contrast to solids, the particles have
some translational kinetic energy, which means they have freedom to move
gas phase - Answer-the phase of matter in which atoms or molecules can move essentially
independently of one another
Which state(s) of matter take the shape of their container? - Answer-Liquids and gases take the shape of
their containers since, they do not have definite shape and volume. Only solids have definite shape and
volume.
SOLIDS - Answer-have the least volume
LIQUIDS - Answer-have a definite volume
GASES - Answer-have maximum volume
Which of the following is correct?
(a) Solids have a definite shape and definite volume.
(b) Liquids have a definite volume but no definite shape.
(c) Gases have a definite volume but no definite shape.
(d) Liquids have both definite shape and definite volume. - Answer-(a) Solids have definite shape and
definite volume.