October 14,2021
Ariana Carter
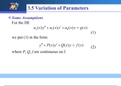
Objective:
To determine the melting point and IR spectrum of benzocaine (ethyl 4-aminobenzoate), benzoic acid and
9-fluorenone using liquid-liquid extraction and recrystallization.
Introduction:
Natural compounds rarely exist without being in a mixture with other compounds. Due to this, chemists
have resorted to various techniques to isolate these compounds and study the various properties associated
with them. In this lab, liquid-liquid extraction was used (as it takes advantage of acids and bases’ ability to
change between a charged and uncharged state and their solubility) in order to separate a mixture containing
the 3 different compounds (benzoic acid, benzocaine and 9-fluorenone). Once the three compounds were
separated through the process of “washing”, the compounds were recrystallized (the process of dissolving
impure solid compounds in a hot solvent and dissolving the impurities, and crystallizing the pure
compound) in order to determine the melting point and IR spectrum.
Procedure:
Refer to the Chemistry 2273 Laboratory Manual I, Fall 2021: pages 94 -107
(Wisner, J. CHEM 2273A Organic Chemistry I Laboratory Manual, Fall 2021. In Western Sciences; UWO
- Graphic Services: London, 2021; p 94-107)
This study source was downloaded by 100000899606070 from CourseHero.com on 09-27-2025 22:00:00 GMT -05:00
https://www.coursehero.com/file/127041670/Experiment-2-2273pdf/
, Results:
Table 1- Crude and purified masses of benzocaine, Benzoic acid and 9-florenone, their %yields and
%recovery
Compound Crude mass(g) Purified mass Percent yield percent yield percent
(g) of crude based of purified recovery of
on weight of based on purified based
mixture (%) weight of on crude(%)
mixture (%)
Benzocaine 0.565* 0.4053* 166.0** 52.4** 31.6**
Benzoic Acid 0.130 0.0536
9-fluorenone 0.852 0.0305
*Due to the benzocaine not crystallizing during the lab, this data was borrowed from Megan Graham in
section 041
**Inaccurate results due to different benzocaine data
Table 2-Qualitative observations, melting points and IR spectra of benzocaine, benzoic acid and 9-
fluorenone
Compound Qualitative Melting points Melting points Literature IR spectra peaks
observations of crude of purified melting points (wavenumber
product(°C) product(°C) (°C) cm-1)
Benzocaine - light 85.3-88.4 87.3-91.0 92 -3340.64(amine
green/yellow group)
~1200s(ester)
-1593.72 (c
double bond in
aromatic ring)
Benzoic acid -solid, larger 88.0-120.0 118.1-121.9 122 -3071.33
crystals, white (carboxyl acid)
and clear -1601.13
9-fluorenone -solid, longer 80.4-82.8 82.4-83.9 84 -1712.80 (ketone
and thin group)
crystals, lime -1595.80
green,
Discussion:
a) Discussion of method:
Liquid-liquid extraction is dependent on the acids and bases’ ability to interchange between their
charged and uncharged states when mixed with strong acids and bases. Due to the nature of polar
and non-polar molecules, it can be suggested that charged compounds are soluble in water, while
uncharged compounds are soluble in organic compounds. In this experiment, a diochloromethane
(DCM) solution was separated into three different compounds using this concept.
To begin, the DCM solution was mixed with HCL to dissolve the Benzocaine into an aqueous layer
(HCL donates protons to benzocaine, turning it into benzocaine hydrochloride). The 9-fluorenone
and benzoic acid remained in a lower organic layer (do not react with HCL).
NaOH was then added to the lower organic layer, thus separating the neutral compound and the
acid ( benzoic acid performs an acid-base reaction with NaOH by donating a proton, forming
sodium benzoate in an aqueous solution, and the 9-fluorenone remained in the lower organic layer
This study source was downloaded by 100000899606070 from CourseHero.com on 09-27-2025 22:00:00 GMT -05:00
https://www.coursehero.com/file/127041670/Experiment-2-2273pdf/