Metamorphism
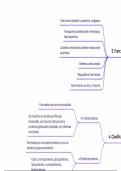
Factors Controlling Metamorphic Processes
Four major factors control metamorphic processes:
• Temperature
• Pressure
• Pore fluid pressure
• Time
The Earth’s Heat
The temperature change ( oC) with depth (km) can be plotted on a graph to give a curve called the geotherm
The Earth’s internal heat comes from:
• Radioactive elements
• E.g. uranium and potassium
Heat Flow – the amount of heat flowing out at the Earth’s surface. It varies around the world because it
depends on the nature and age of the rocks in any particular area
Pressure in the Earth
The main type of pressure in the Earth’s crust is confining pressure – directly related to depth and may be
thought of as acting vertically, sometimes referred to as vertical pressure or load pressure
Pressure = perpendicular force/area
At any given depth the weight of the overlying rocks will exert pressure on the rocks beneath. We can calculate
the pressure at any depth using the equation:
Pressure = hpg h = depth p = density g = acceleration due to gravity
Pore Fluid Pressure
Fluid – a liquid or gas, they are mobile and can move through the pore spaces in rocks e.g.:
• Water (H2O)
• Carbon dioxide (CO2)
• Sulphur dioxide (SO 2)
• Chlorine (Cl2)
The fluids are found in most rocks, particularly in sediments. The fluids can be in:
• Pore spaces between grains
• Tiny cracks and fractures
• Veins
During deformation and metamorphism pore fluids are heated and compressed. They are under the same
pressure as the rocks themselves but are capable of exerting their own pressure within the pore spaces (pore
fluid pressure). This is important in metamorphism because:
• Pore fluids act as catalysts and accelerate metamorphic reactions
• Pore fluids act as solvents and so bring different ions into contact with each other as they move
Pore fluids pressure is also responsible for the spectacular blow-outs that sometimes occur when oil wells are
drilled.
, Stress and Strain in the Earth’s Crust
Differential Stress – the difference between the maximum and minimum principal stresses. When new
minerals recrystallise during metamorphism, they tend to grow related to these stresses
Three important points to remember:
• Platy minerals (e.g. mica and chlorite) grow perpendicular to the maximum stress direction. If the
differential stress is high, they will grow parallel to the Pmin, e.g. forming slaty cleavage
• Differential stress in fault planes is usually quite low, so new minerals tend to grow parallel to
shearing direction i.e. with the movement
• When folds form the differential stress can be much greater so new minerals (particularly platy ones)
tend to grow parallel to Pmin i.e. parallel to the fold axis
The Significance of Time
Time is important because it varies between different types of metamorphism
Metamorphic Environments
Metamorphism can be categorised as four main metamorphic environments based on temperature and
pressure regimes:
• Contact – temperature changes only
• Regional – pressure and temperature changes
• Burial – pressure and temperature changes
• Dynamic – temperature changes, very little pressure change
Chemical Equilibrium Changes During Metamorphism
Important Information to Remember:
• Increases in pressure and temperature are factors responsible for chemical equilibrium changes
• Metamorphic reactions take place in the solid state (the rock never melts)
• Metamorphism is an isochemical process – the chemistry of the rock does not change, and no new
elements are added
Parent Rock Parent Minerals Metamorphic Rock
Spotted Rock, Hornfels, Schist,
Shale/Mudstone Clay minerals
Gneiss, Slate
Sandstone Quartz Metaquartzite
Limestone Calcite Marble
Basalt Olivine, Augite, Plagioclase Eclogite
Quartz, Orthoclase, Mica Similar to Gneiss
Granite
(Hornblende, Plagioclase)
Real Examples of Equilibrium Changes
Shale
A common clay in shale is kaolinite and it has the chemical formula Al2Si2O5(OH)4. Since clay minerals are
hydrated aluminium silicates, when they react oxides (e.g. H2O, Al2O3 and SiO2) are created. Out of these 3
compounds H2O will be lost during metamorphism but Al2O3 and SiO2 will form new minerals.
Factors Controlling Metamorphic Processes
Four major factors control metamorphic processes:
• Temperature
• Pressure
• Pore fluid pressure
• Time
The Earth’s Heat
The temperature change ( oC) with depth (km) can be plotted on a graph to give a curve called the geotherm
The Earth’s internal heat comes from:
• Radioactive elements
• E.g. uranium and potassium
Heat Flow – the amount of heat flowing out at the Earth’s surface. It varies around the world because it
depends on the nature and age of the rocks in any particular area
Pressure in the Earth
The main type of pressure in the Earth’s crust is confining pressure – directly related to depth and may be
thought of as acting vertically, sometimes referred to as vertical pressure or load pressure
Pressure = perpendicular force/area
At any given depth the weight of the overlying rocks will exert pressure on the rocks beneath. We can calculate
the pressure at any depth using the equation:
Pressure = hpg h = depth p = density g = acceleration due to gravity
Pore Fluid Pressure
Fluid – a liquid or gas, they are mobile and can move through the pore spaces in rocks e.g.:
• Water (H2O)
• Carbon dioxide (CO2)
• Sulphur dioxide (SO 2)
• Chlorine (Cl2)
The fluids are found in most rocks, particularly in sediments. The fluids can be in:
• Pore spaces between grains
• Tiny cracks and fractures
• Veins
During deformation and metamorphism pore fluids are heated and compressed. They are under the same
pressure as the rocks themselves but are capable of exerting their own pressure within the pore spaces (pore
fluid pressure). This is important in metamorphism because:
• Pore fluids act as catalysts and accelerate metamorphic reactions
• Pore fluids act as solvents and so bring different ions into contact with each other as they move
Pore fluids pressure is also responsible for the spectacular blow-outs that sometimes occur when oil wells are
drilled.
, Stress and Strain in the Earth’s Crust
Differential Stress – the difference between the maximum and minimum principal stresses. When new
minerals recrystallise during metamorphism, they tend to grow related to these stresses
Three important points to remember:
• Platy minerals (e.g. mica and chlorite) grow perpendicular to the maximum stress direction. If the
differential stress is high, they will grow parallel to the Pmin, e.g. forming slaty cleavage
• Differential stress in fault planes is usually quite low, so new minerals tend to grow parallel to
shearing direction i.e. with the movement
• When folds form the differential stress can be much greater so new minerals (particularly platy ones)
tend to grow parallel to Pmin i.e. parallel to the fold axis
The Significance of Time
Time is important because it varies between different types of metamorphism
Metamorphic Environments
Metamorphism can be categorised as four main metamorphic environments based on temperature and
pressure regimes:
• Contact – temperature changes only
• Regional – pressure and temperature changes
• Burial – pressure and temperature changes
• Dynamic – temperature changes, very little pressure change
Chemical Equilibrium Changes During Metamorphism
Important Information to Remember:
• Increases in pressure and temperature are factors responsible for chemical equilibrium changes
• Metamorphic reactions take place in the solid state (the rock never melts)
• Metamorphism is an isochemical process – the chemistry of the rock does not change, and no new
elements are added
Parent Rock Parent Minerals Metamorphic Rock
Spotted Rock, Hornfels, Schist,
Shale/Mudstone Clay minerals
Gneiss, Slate
Sandstone Quartz Metaquartzite
Limestone Calcite Marble
Basalt Olivine, Augite, Plagioclase Eclogite
Quartz, Orthoclase, Mica Similar to Gneiss
Granite
(Hornblende, Plagioclase)
Real Examples of Equilibrium Changes
Shale
A common clay in shale is kaolinite and it has the chemical formula Al2Si2O5(OH)4. Since clay minerals are
hydrated aluminium silicates, when they react oxides (e.g. H2O, Al2O3 and SiO2) are created. Out of these 3
compounds H2O will be lost during metamorphism but Al2O3 and SiO2 will form new minerals.