Bioenergetics of exercise
• Bioenergetics – study of energy transfer from food to useable energy
• Energy transfer occurs via the release of energy in chemical bonds (large storage of potential
energy)
• Using energy to power repeated muscle contractions
Coupled reactions
• One reaction will release (free) energy and this will be used to power another
o Endergonic and exergonic reactions – exergonic powers your endergonic reactions
• Endergonic – a reaction that requires energy
• Exergonic – a reaction that releases free energy
• Example: catabolism – breakdown of sugars into glucose through cellular metabolism, glucose is
broken down further to give useable energy
• Oxidation-reduction reactions are important
o Oxidation = removal of electrons
o Reduction = addition of electrons
o Transfer from one molecule to another = oxidation-reduction reaction
o Allows occurs together; one thing cannot be oxidised without reducing another
Enzymes
• Biological catalysts that enhance that rate of chemical reactions
• Enzymes do not cause reactions to occur, they rather control the rate at which it happens
• Can be upregulated (activators) or downregulated (inhibitors)
• Important in regulating exercise metabolism
• A catalysed reaction makes it easier for a reaction to take place, therefore it can happen more
rapidly
• Example: lipase (lipids), lactate dehydrogenase (lactate)
• Can be used as diagnostic tools – high levels of enzymes = certain diseases
o E.g. creatine kinase found in patients suffering from myocardial infarction
Fuels for exercise
a. Carbohydrates
• Stored CHO provides the body with its most usable form of energy
• 1g = 4kcal of (useable) energy
• Monosaccharides – the most, easily absorbed by the gut e.g. glucose and fructose
• Disaccharides – e.g. sucrose, first must be broken down to be used for energy
• Polysaccharide – e.g. glycogen: stored glucose in animal tissue, stored in the muscle and liver
o Broken down into glucose by glycogenolysis
b. Fats
• Found in animals, plants
• Higher carbon count than carbohydrates, therefore require more oxygen for oxidation
• 1g = 9 kcal of energy
, • E.g. fatty acids, triglycerides, phospholipids, steroids
• Fatty acids used for long-duration exercise, excess is stored as triglycerides
o Stored in muscle cells and adipose tissue
o TGs are broken down into FFA through lipolysis
• Cholesterol is important in maintaining cell lining, and producing hormones
• Too high levels can lead to cardiovascular disease
• Unsaturated fats – help remove bad cholesterol and maintain good cholesterol
o E.g. fatty fish, avocado, nuts, olive oil (good), animal fats, fried fatty foods (bad)
c. Proteins
• Proteins do not play a large role in exercise endurance, they are important in maintaining one’s
muscle mass
• Broken down into amino acids
o 9 are essential because the body cannot create them itself
• 1g = 4kcal of energy
• Proteins can be converted into glucose ® energy (during exercise)
• Found in animals and plants e.g. lentils, chicken, meat, fish, dairy, eggs
Adenosine Triphosphate
• ATP = adenine, ribose and 3 phosphate molecules
• Macronutrients need to be broken down into useable energy
• Synthesis of ATP requires energy ® obtained from the catabolism of our nutrients
• ATP ® ADP + Pi
• Splitting of ATP forms free energy which is used for different actions (muscle contractions)
• ATPase promotes the splitting of ATP ®® 7.3 kcal of energy for work
• In recovery, ATP is resynthesized by joining ADP and Pi
• The body only stores small amounts of ATP (90 g)
• During activity, we rely on rapid regeneration of ATP ® energy pathways
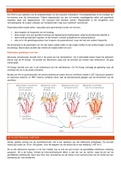
Energy pathways