Solutions and Aqueous Reactions
Lecture Notes
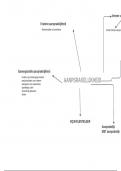
mass (g)
*
mol ⑭mol/L conversion
molar mass volume set up
calculating
Solution Concentration Practice Problem 8 I .
the of by adding 45 4g of NaNO3 to create volume
calculating molarity a solution made .
of total 2 SOL
.
NaNOz molar mass
45 /mol NaNOs
M =
45 .
49
.
4g = 0 21 L
.
23 +14 +
16(3)
859 85g/mol
-
1) ol
=
2 50L 2 501
-
=
.
.
Practice Problems 8 .
2 Using Molarity in Calculations
how L of 0 125 M NaOH solution contain O 255 mol NaOH ?
many a . .
molarity
=
0 .
125 --
0 125.
mol/L
0 255 mol NaOH
. 14 - 2 04 L
.
-
0 125 mol NaOH
.
how many grams of sucrose are in 1 55L
.
of 0 758 M
. sucrose solution ?
sucrose : CH220 , "molar mass
=
12(12) + 22 + 16(11) = 342 g/mol
1 551
.
0 758mol
.
3429 = 401 8 .
+
4029
14 1 mol z
Practice Problem 8 3 .
to what volume should
you dilute 0 2001
.
of a 15 O M
.
NaOH solution to obtain a 3 00 M NaOH
.
solution ?
M
, =
13 0 M NaOH 15 0 M (0 200L)
Ve
. .
.
=
v, =
0 200L
. 3 00 M
.
My = 3 00 M NaOH
. Vz =
1 00 L
.
2
Vz =?
Lecture Notes
mass (g)
*
mol ⑭mol/L conversion
molar mass volume set up
calculating
Solution Concentration Practice Problem 8 I .
the of by adding 45 4g of NaNO3 to create volume
calculating molarity a solution made .
of total 2 SOL
.
NaNOz molar mass
45 /mol NaNOs
M =
45 .
49
.
4g = 0 21 L
.
23 +14 +
16(3)
859 85g/mol
-
1) ol
=
2 50L 2 501
-
=
.
.
Practice Problems 8 .
2 Using Molarity in Calculations
how L of 0 125 M NaOH solution contain O 255 mol NaOH ?
many a . .
molarity
=
0 .
125 --
0 125.
mol/L
0 255 mol NaOH
. 14 - 2 04 L
.
-
0 125 mol NaOH
.
how many grams of sucrose are in 1 55L
.
of 0 758 M
. sucrose solution ?
sucrose : CH220 , "molar mass
=
12(12) + 22 + 16(11) = 342 g/mol
1 551
.
0 758mol
.
3429 = 401 8 .
+
4029
14 1 mol z
Practice Problem 8 3 .
to what volume should
you dilute 0 2001
.
of a 15 O M
.
NaOH solution to obtain a 3 00 M NaOH
.
solution ?
M
, =
13 0 M NaOH 15 0 M (0 200L)
Ve
. .
.
=
v, =
0 200L
. 3 00 M
.
My = 3 00 M NaOH
. Vz =
1 00 L
.
2
Vz =?