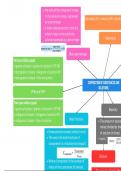
the ratio of the component’s mass
to the solution’s mass, expressed
Normality (N) = molarity (M) × electro
as a percentage
A mass-volume percent: ratio of a
solute’s mass to the solution’s
volume expressed as a percentage Normality
Mass percentage
Parts per billion (ppb)
= (grams of solute ÷ grams of solution) × 10⁹ OR
= microgram of solute ÷ kilogram of solution OR
= microgram of solute ÷ litre of solution
COMPOSITION OF SUBSTANCES AND
SOLUTIONS
PPM and PPB
Parts per million (ppm)
= (grams of solute ÷ grams of solution) × 10⁶ OR Molarity
= milligram of solute ÷ kilogram of solution OR
= milligram of solute ÷ litre of solution Mole fraction The amount of solute
moles) divided by the
Dimensionless number (without units). of solution (in litres)
The sum of all mole fractions of mol.L-¹
components in a solution must equal 1
Dilution
Where ncomponent is the number of
moles of the component of interest,
to the solution’s mass, expressed
Normality (N) = molarity (M) × electro
as a percentage
A mass-volume percent: ratio of a
solute’s mass to the solution’s
volume expressed as a percentage Normality
Mass percentage
Parts per billion (ppb)
= (grams of solute ÷ grams of solution) × 10⁹ OR
= microgram of solute ÷ kilogram of solution OR
= microgram of solute ÷ litre of solution
COMPOSITION OF SUBSTANCES AND
SOLUTIONS
PPM and PPB
Parts per million (ppm)
= (grams of solute ÷ grams of solution) × 10⁶ OR Molarity
= milligram of solute ÷ kilogram of solution OR
= milligram of solute ÷ litre of solution Mole fraction The amount of solute
moles) divided by the
Dimensionless number (without units). of solution (in litres)
The sum of all mole fractions of mol.L-¹
components in a solution must equal 1
Dilution
Where ncomponent is the number of
moles of the component of interest,