Page # 1
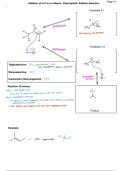
Addition of H-X to an Alkene - Electrophilic Addition Reaction
Possibility # 1
H H
H
protonation CHz cuz
H C H
C C notformed toounstable
H
H H
Possibility # 2
H
ABr protonation
H
CH
Regioselective:
YES
4
mayoknewnioknfinitionaleisomer
issavored
Stereoselective: NO
nucleophilic Br
Carbocation Rearrangement: YES attack
Reaction Summary:
Step1 PROTONATION aBoor
It goes to carbon of doublebond with mostprotons
Step 2 NUCLEOPHILIC ATTACK
x goestothemostcarbocation like carbocation followingcarbocation
rearrangement it necessary
the halogengetsadded on to the more substitutedcarbon
Product
Example:
+ HCl CII
, Page # 2
Acid Catalyzed Hydration of an Alkene
O H O
H O S O H
+f O proton
O + O S O H
H H transfer
O
µ H H
O
H
H C H protonation zo H2O
C C
H
H
y H
H
GO
H
H
nucleophilic
attack
4 H O H
oo H H
iOH H O H I
0
I
deprotonation
I Oxonium
ion
Product
Reaction Summary:
Step 1 protonation of alkene to generate more stable
electrophilic carbocation
moleculeon
Step 2 attackof thenucleophilicH2O
createsan oxoniumion
Regioselectivity:
YES Markovnikov
alcohol
step3 deprotonation by abasegeneratesthe
OHGOESONMORE SUBSTITUTEDCARBON
FOLLOWSMARKOVNIKOV Stereoselectivity:NO racemic mixture
RACEMICMIXTURE FORMED IF THERE'S ACHIRALCENTER
Example: Carbocation Rearrangement:YES
OH
Cat H 2SO 4
N
H 2O racemic
Addition of H-X to an Alkene - Electrophilic Addition Reaction
Possibility # 1
H H
H
protonation CHz cuz
H C H
C C notformed toounstable
H
H H
Possibility # 2
H
ABr protonation
H
CH
Regioselective:
YES
4
mayoknewnioknfinitionaleisomer
issavored
Stereoselective: NO
nucleophilic Br
Carbocation Rearrangement: YES attack
Reaction Summary:
Step1 PROTONATION aBoor
It goes to carbon of doublebond with mostprotons
Step 2 NUCLEOPHILIC ATTACK
x goestothemostcarbocation like carbocation followingcarbocation
rearrangement it necessary
the halogengetsadded on to the more substitutedcarbon
Product
Example:
+ HCl CII
, Page # 2
Acid Catalyzed Hydration of an Alkene
O H O
H O S O H
+f O proton
O + O S O H
H H transfer
O
µ H H
O
H
H C H protonation zo H2O
C C
H
H
y H
H
GO
H
H
nucleophilic
attack
4 H O H
oo H H
iOH H O H I
0
I
deprotonation
I Oxonium
ion
Product
Reaction Summary:
Step 1 protonation of alkene to generate more stable
electrophilic carbocation
moleculeon
Step 2 attackof thenucleophilicH2O
createsan oxoniumion
Regioselectivity:
YES Markovnikov
alcohol
step3 deprotonation by abasegeneratesthe
OHGOESONMORE SUBSTITUTEDCARBON
FOLLOWSMARKOVNIKOV Stereoselectivity:NO racemic mixture
RACEMICMIXTURE FORMED IF THERE'S ACHIRALCENTER
Example: Carbocation Rearrangement:YES
OH
Cat H 2SO 4
N
H 2O racemic