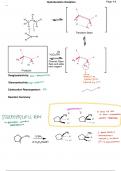
Hydroboration Oxidation Page # 9
H
i
H i
t
H
C
C
C
H aFf
H H
f
H H
H g B H
Transition State
H
H H
i i
H 2O2/OH
H H
cµzft cµzffI
H AH Chemist Open
flask and adds H 1At
OH new reagent B
Products
t
Reagioselectivity: Markovnikov
Non Ye'Yabated
by
hydroxylgroup
replacedby OH
Stereoselectivity:
syn addition
OH
Carbocation Rearangement: NO
Reaction Summary:
enantiomers
I µ and OH are
in anti orientation
STEREOSPECIFIC RYN
TIKI p EI NEVER OBSERVED
generates certain
stereoisomers
ng It
CH 3 1) BH 3
i
2) H 2O2/NaOH 22 4 stereosiomers
H
i
H i
t
H
C
C
C
H aFf
H H
f
H H
H g B H
Transition State
H
H H
i i
H 2O2/OH
H H
cµzft cµzffI
H AH Chemist Open
flask and adds H 1At
OH new reagent B
Products
t
Reagioselectivity: Markovnikov
Non Ye'Yabated
by
hydroxylgroup
replacedby OH
Stereoselectivity:
syn addition
OH
Carbocation Rearangement: NO
Reaction Summary:
enantiomers
I µ and OH are
in anti orientation
STEREOSPECIFIC RYN
TIKI p EI NEVER OBSERVED
generates certain
stereoisomers
ng It
CH 3 1) BH 3
i
2) H 2O2/NaOH 22 4 stereosiomers