with Questions and Correct Answers| Latest Update |
Verified
What is Turbidity? - ✔✔Turbidity can be described as a measure of the relative clarity
of water. Turbidity is an expression of the optical property that causes light to be
scattered and absorbed rather than transmitted in straight lines through the sample.
What causes Turbidity? - ✔✔Turbidity is caused by clay, slit, finely divided organic
and inorganic material. Other suspended matter and microscopic organisms can also
cause turbidity. There is one approved method for running turbidity - the Nephelometric
Method (NTU)
What is chlorine Demand? - ✔✔The amount of chlorine used up to completely react
with the water and its suspended or dissolve material
What is chlorine residual? - ✔✔When all the demand of the water is met any
additional chlorine produces a chlorine residual
What is chlorine dosage? - ✔✔Demand plus residual is the chlorine dosage. Chlorine
Dosage = Demand + Residual
What determines rate of disinfection? - ✔✔Chlorine concentration and contact time
determines the rate and degree of disinfection. If concentration increases, the time can
be reduced; if concentration is reduced the time must be increased.
How does temperature effect chlorine effectiveness? - ✔✔Chlorine effectiveness is
greater at higher temperatures, up to the point that chlorine volatizes. At low
temperature, chlorine is more stable, but disinfection time increases.
,Is removing turbidity important for disinfection? - ✔✔Turbidity must be removed to
low levels by sedimentation and filtration to allow chlorine to contact pathogens.
What happens to dissolved solids on contact with chlorine? - ✔✔Dissolved solids are
oxidized on contact further reinforcing the importance of contact time.
No residual is formed until reducing agents are destroyed. What are examples of
reducing agents? - ✔✔Manganese, iron, turbidity, organic matter, ammonia, or
nitrates
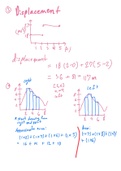
What the 4 stages of the Chlorine Demand Curve chart? - ✔✔Stage 1: No residual is
formed because inorganic demand destroys the chlorine. (Flat Line)
Stage 2: Monochloramines are formed as chlorine combines with organics containing
ammonia (rising curve). The chlorine to ammonia weight ratio at this point is 5:1.
Combined and total residual increases, chloro-organics are formed.
Stage 3: Increasing chlorine dosage (failing curve) to a chlorine/ammonia weight ratio of
7.6:1 destroys chloramines and chloro-organics. Combined decreases and free chlorine
increases.
Stage 4: Enough chlorine is added to reduce all demand. The combined residual remains
the same, but free residual increases with dosage. This stage is called "breakpoint"
chlorination. The chlorine to ammonia ratio of 10:1 satisfies all demand and disinfects
the distribution system. ( second rising curve)
What is the difference between free chlorine and combined chlorine
(chloramines/chlorine-ammonia) systems? - ✔✔Free chlorine is more powerful than
combined chlorine, but combined chlorine lasts longer.
,- combined chlorine requires a 60-minute detention time while free chlorine requires 10
minutes.
-If combined residual is replaced by a free residual, less taste and odor in the water
usuals occurs.
- if combined residual is used : ph and temperature must be monitored closely and the
residual adjusted accordingly. For example, as the temperature decreases the combines
residual must be increased. Free residual does not require such monitoring in the 6.0 -
8.0 pH range. Free chlorine residual concentration is not affected by temperature
changes.
What is the most important factor affecting the useful life of service lines? - ✔✔It is
the ability of the material to resist internal and external corrosion
When does Suction Head exist? - ✔✔Suction head exists when the source of supply is
above the centerline of the pump.
What is Net positive Suction Head (NPSH) - ✔✔NPSH is the pressure under which
water enters the eye of the impeller in a centrifugal pump. Insufficient NPSH is the main
cause of pump cavitation
Explain and expand on Chlorine Dioxide (what it reacts with, oxidizes, etc) -
✔✔Chlorine Dioxide is a gas used in small quantities to disinfect water. It does not
react with organics to form THMs and HAAs. It oxidizes phenols, manganese, iron,
sulfurous and organic compounds which aids in the removal of tastes and odors. It is a
very effective bactericide and a superior virocide. It dies not combine with ammonia.
Chlorine Dioxide MRDL is .8 mg/L and monitoring procedures are the same as for
chlorine. The Chlorite MCL is .q mg/L and must be monitored daily.
What is texas drinking water pH requirements? - ✔✔The texas drinking water
requirements require a pH > 7.0 for the water treatment plant finished water. EPA
requires that pH analysis be preformed within 15 minutes of the sample being caught.
, How does pH affect Chlorine activity? - ✔✔When chlorine is added to water it forms
hypochlorous acid (HOCL) and hydrochloric acid (OCL). The hypochlorous acid is the
effective disinfectant. At a pH of 4.0 there is 100% hypochlorous acid. At a pH of 11
there is 100% hypochlorite ion. At a pH of 7, the hypochlorous acid is at about 80% and
the hypochlorite ion makes up about 20% of the chlorine content. The lower the pH is
more effective chlorine is because you have more hypochlorous acid.
What is meant by water stability? - ✔✔Stable water is defined as water that will
neither deposit or dissolve a calcium carbonate film or scale. Since no water is stable
indefinitely, chemical treatment of water usually is intended to produce water that is
slightly scale forming without being liable to cause stoppages in small lines and services.
Bayliss curve is the simplest determination of stability. The Langelier saturation index is
also used.
What/how are above ground tanks and piping protected from corrosion? -
✔✔Corrosion protection for above ground tanks and piping is prevented by: cathodic
protection, use if protective linings/coatings, eliminating different types of metals in the
distribution system, and treating the water with chemicals to make it less corrosive
How do you tests fir alkalinity? - ✔✔The two tritiation methods of analysis are a
standard acid to titrate a sample. The end point of the titration is determined by the
following: an indicator that changes color at a certain pH, or potentiometric titration to
end - point pH. Using either of the methods, a measured amount of sample is titrated
with a standard sulfuric acid to a predetermined pH. Nomograph are a graphical form of
determining alkalinity. If the pH, total alkalinity, temperature and total dissolved solids
are known, any or all of the alkalinity forms may be estimated.
What is the minimum chlorine residual at the far reaches of the system for both free
and combined? - ✔✔Free chlorine residual = .2mg/L