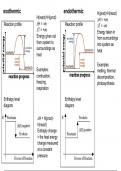
H(react)>H(prod) ∆H = +ve
Reaction profile ∆H = -ve Reaction profile ∆T = -ve
∆T = +ve Energy taken in
Energy given out from surroundings
from system to into system as
surroundings as heat
heat
Examples:
Examples: melting, thermal
combustion, decomposition,
freezing, photosynthesis
respiration
Enthalpy level Enthalpy level
diagram diagram
∆H = H(prod)-
H(react)
Enthaply change
= the heat energy
change measured
at a constant
pressure.