Introduction
What is viscosity?
Viscosity is the resistance of a fluid to flow present in viscous fluids. There are two types of viscosity:
static viscosity and dynamic viscosity. Dynamic viscosity is the measurement of the fluid's internal
resistance to flow while static viscosity is the viscosity of a stationary fluid. (Nieto, 2019)
Viscosity is affected by a variety of things including temperature, pressure and dilution. These will be
discussed in turn below:
Temperature
Liquids, such as water, have a low viscosity. Cold liquids have a high viscosity and hot liquids have a
low viscosity. As the temperature increases, the viscosity increases. This is because intermolecular
forces between the molecules weaken due to the temperature increasing the kinetic energy, which
decreases the viscosity. (Bing.com, 2019)
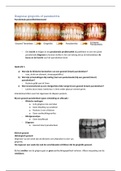
Liquid graph
(Kleo.bergdorfbib.co, 2019)
Fig.1 Dynamic viscosity compared to temperature for water graph.
This graph shows the trend that as the temperature of the water increases, the viscosity of the fluid,
water, decreases.
Pressure
Increase of pressure on fluid has been known to increase the viscosity of a substance. This Is because
the amount of free volume in the internal structure decreases due to compression. Therefore, the
molecules can move less freely, and the internal friction forces increase.
Dilution
Additions of a solute reduces the viscosity of the solvent. This is because as the fluid becomes more
diluted and thinner. The fluid can flow easier and becomes less viscous.