Pearson Edexcel Mark Scheme (Results) Summer 2022 Pearson Edexcel GCSE In Chemistry (1CH0) Paper 2H Mark Scheme (Results) Summer 2022 Pearson Edexcel GCSE In Chemistry (1CH0) Paper 2H Edexcel and BTEC Qualifications
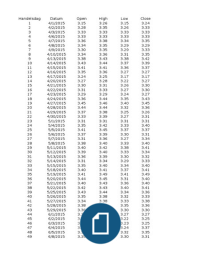
Pearson Edexcel Mark Scheme (Results) Summer 2022 Pearson Edexcel GCSE In Chemistry (1CH0) Paper 2H Mark Scheme (Results) Summer 2022 Pearson Edexcel GCSE In Chemistry (1CH0) Paper 2H Edexcel and BTEC Qualifications Edexcel and BTEC qualifications are awarded by Pearson, the UK’s largest awarding body. We provide a wide range of qualifications including academic, vocational, occupational and specific programmes for employers. For further information visit our qualifications websites at or . Alternatively, you can get in touch with us using the details on our contact us page at Pearson: helping people progress, everywhere Pearson aspires to be the world’s leading learning company. Our aim is to help everyone progress in their lives through education. We believe in every kind of learning, for all kinds of people, wherever they are in the world. We’ve been involved in education for over 150 years, and by working across 70 countries, in 100 languages, we have built an international reputation for our commitment to high standards and raising achievement through innovation in education. Find out more about how we can help you and your students at: Summer 2022 Publications Code 1CH0_2H_2206_MS All the material in this publication is copyright © Pearson Education Ltd 2022 General Marking Guidance • All candidates must receive the same treatment. Examiners must mark the first candidate in exactly the same way as they mark the last. • Mark schemes should be applied positively. Candidates must be rewarded for what they have shown they can do rather than penalised for omissions. • Examiners should mark according to the mark scheme not according to their perception of where the grade boundaries may lie. • There is no ceiling on achievement. All marks on the mark scheme should be used appropriately. • All the marks on the mark scheme are designed to be awarded. Examiners should always award full marks if deserved, i.e. if the answer matches the mark scheme. Examiners should also be prepared to award zero marks if the candidate’s response is not worthy of credit according to the mark scheme. • Where some judgement is required, mark schemes will provide the principles by which marks will be awarded and exemplification may be limited. • When examiners are in doubt regarding the application of the mark scheme to a candidate’s response, the team leader must be consulted. • Crossed out work should be marked UNLESS the candidate has replaced it with an alternative response. Mark schemes have been developed so that the rubrics of each mark scheme reflects the characteristics of the skills within the AO being targeted and the requirements of the command word. So for example the command word ‘Explain’ requires an identification of a point and then reasoning/justification of the point. Explain questions can be asked across all AOs. The distinction comes whether the identification is via a judgment made to reach a conclusion, or, making a point through application of knowledge to reason/justify the point made through application of understanding. It is the combination and linkage of the marking points that is needed to gain full marks. When marking questions with a ‘describe’ or ‘explain’ command word, the detailed marking guidance below should be consulted to ensure consistency of marking. Assessment Objective Command Word Strand Element Describe Explain AO1* An answer that combines the marking points to provide a logical description An explanation that links identification of a point with reasoning/justification(s) as required AO2 An answer that combines the marking points to provide a logical description, showing application of knowledge and understanding An explanation that links identification of a point (by applying knowledge) with reasoning/justification (application of understanding) AO3 1a and 1b An answer that combines points of interpretation/evaluation to provide a logical description AO3 2a and 2b An explanation that combines identification via a judgment to reach a conclusion via justification/reasoning AO3 3a An answer that combines the marking points to provide a logical description of the plan/method/experiment AO3 3b An explanation that combines identifying an improvement of the experimental procedure with a linked justification/reasoning *there will be situations where an AO1 question will include elements of recall of knowledge directly from the specification (up to a maximum of 15%). These will be identified by an asterisk in the mark scheme. 1CH0/2H 2206 Paper 2 Higher Tier Question number Answer Additional guidance Mark 1(a)(i) colourless / absorbs UV / non-toxic / large SA : vol ratio allow transparent / does not leave white marks allow reflects UV (1) AO2 1 Question number Answer Additional guidance Mark 1(a)(ii) long term effects not known/ may build up in {living things/ water supplies/ environment} allow specific examples of effects on health but ignore ‘health risks’ allow may get into the body and cause harm (1) AO1 1 Question number Answer Additional guidance Mark 1(b)(i) as the diameter of the nanoparticle increases the surface area volume ratio decreases ORA allow negative correlation/inversely proportional ignore that as volume increases surface area also increases (1) AO3 1 Question number Answer Mark 1(b)(ii) B 3 : 40 is the only correct answer. A is the correct ratio for a 70nm diameter sphere C is the correct ratio for a 90nm diameter sphere D is the correct ratio for a 100nm diameter sphere (1) AO3 1 Question number Answer Additional guidance Mark 1(c) calculate surface area 60 x 60 x 6 (= 21 600 ) (1) calculate volume 60 x 60 x 60 (= 216 000 ) (1) s.a : vol ratio (1) (= 10) 21600 allow 10 : 1 (or multiples of) with calculation ignore = instead of : (3) AO2 1 (Total for question 1 = 7 marks) Question number Answer Additional Guidance Mark 2(a)(i) • 100 cm3 measuring cylinder/ (gas) syringe (1) • which has smaller gradations / higher resolution (1) allow ‘smaller measuring cylinder’ ignore gas measurer reject (upturned) burette for MP1 MP2 is dependent on MP1 allow (more) precise / (more) accurate allow smaller measurements/ increments ignore easier to use / no gas will escape (2) AO3 3b Question number Answer Additional guidance Mark 2(a)(ii) • volume read at 90s = 29 cm3 (1) • rate = volume (1) 90 • = 0.3222…. (cm3 per second) (1) 0.31, 0.32, 0.33 with or without working scores 3 0.3 alone scores 0 all other answers require working to have marks awarded allow any value 28-30 ECF for incorrect volume ECF if fraction inverted ECF if 1.5 used instead of 90 eg 28/29/30 = 18.66…/ 19.33…/ 20 scores 2 1.5 MP3 must be decimal value correctly rounded – ignore fractions (3) AO3 2 Question number Answer Additional guidance Mark 2(a)(iii) volumes were {constant / stopped rising} OR graph was {flat/plateaued/ levelled off} allow reactant(s) used up / limiting factor allow no more hydrogen evolved allow EVIDENCE that reaction stopped: measurements stayed the same/ no more bubbles allow graph has reached zero gradient ignore graph is a straight line ignore it has reached the highest {point / volume} ignore reaction has stopped / is complete reject reaction is becoming slower (1) AO3 2 Question number Answer Additional guidance Mark 2(b)(i) An explanation linking • more particles present (in same volume) (1) • so more frequent collisions/ more chance of collision (1) allow atoms/ molecules/ ions for particles ignore more acid present allow more collisions per {sec/min/unit time} ignore more collisions/ more successful collisions ignore references to energy / moving faster mark independently (2) AO1 1 Question number Answer Mark 2(b)(ii) D use the same metal but in a powdered form is the only correct answer B and C are incorrect because the reactants are not changed A is incorrect because the reaction will be slower (1) AO2 1 (Total for question 2 = 9 marks) Question number Answer Mark 3(a) B effervescense is seen is the only correct answer. A, C and D are incorrect as they are not linked to gas production (1) AO1 2 Question number Answer Mark 3(b) B chlorine is the only correct answer. A, C and D are incorrect because only chlorine bleaches litmus. (1) AO1 1 Question number Answer Additional guidance Mark 3(c) 2.20 with or without working scores (2) • 5(.000) – 2.8(00) = 2.2(00) (1) • = 2.20 (1) reject additional processing for MP1 (e.g 5 – 2.8 = 2.2 then 2.2 = 0.0220) 100 does not score MP1 – additional process of dividing by 100 does not score MP2 - using a number not in the question for MP2 final answer must be to 3sf, correct evaluation of expression using only numbers from the question 2.2 / 2.200 scores 1 mark 5.000 = 1.79 scores 1 mark 2.800 2.800 = 0.560 scores 1 mark [0.56 = 0] 5.000 5.000 x 2.800 = 14.0 scores 1 mark [14 = 0] 5.000 + 2.800 = 7.80 scores 1 mark [7.8 = 0] (2) AO2 1 Question number Answer Additional guidance Mark 3(d)(i) An explanation linking: • it has two electrons in outer shell/ it has a full outer shell / OWTTE (1) • so does not {gain/ lose/ transfer/ share} electrons (1) MP1 – reject if number of electrons in outer shell is stated and not 2 ignore references to protons and neutrons allow helium has two electrons in its (only) shell / helium’s (only) shell is full ignore helium does not need to react (2) AO1 1 Question number Answer Additional guidance Mark 3(d)(ii) less dense than air allow less dense than nitrogen allow low density / not (very) dense allow diffuses slowly out of balloon ignore less dense than oxygen ignore it is a gas / light / lightweight / inert/ unreactive/ non-flammable / lighter than air / makes balloon float / it rises/ it floats ignore non-toxic / not poisonous (1) AO2 1 Question number Answer Additional guidance Mark 3(e) 4.214 x 1024 with or without working scores (2) 2 x 3.5 (1) (= 7(.0)) 7(.0) x 6.02 x 1023 (1) (= 4.214 x 1024 ) OR 3.5 x 6.02 x 1023 (1) (= 2.107 x 1024) 2 x 2.107 x 1024 (1) (= 4.214 x 1024) allow any number of sig figs except 1 for full marks allow answer no
Written for
Document information
- Uploaded on
- July 30, 2023
- Number of pages
- 29
- Written in
- 2022/2023
- Type
- Exam (elaborations)
- Contains
- Questions & answers
Subjects
-
pearson edexcel mark scheme results summer 2022