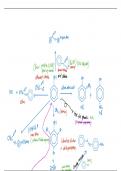
Éo gttbotival
x
Base pyridinesHsn Yagatl
Benzoylchloride
lessreactive
speedupreaction
OH acyl
chloride
ethanol chloride on
Na
Ha CHIEF ftbÉd dilutenitrigacid
t
ester
gBrafranset 70 Nos
test
forphenols Fed
OH it producesapurplesolution
Br Br
to
c
c.si moreoften
f
Colourlesssolution
whiteprecipitate
NIN NHI
Br T
t ftp.litgqgene.coaplingreat it
, benzenediazonium chloride
gg
ftNatal NENCT
fidN alkalinesolution
x
diazotisation
excess nitrousacid at ie
it below lot it would bestable
OH unilatalititaticamine which
yellowppt if haveNaoly
ng need include thosetoo
so Nacl H2O very
unstableandbecome alcohol immediately
brijeg
NH d
dye Nth
acid base
NaOH liberatethe
phenylamine
phenylamine
Reduction f
Collect by steamdistillation
agent in water
thesolubility of
Reducing phenylamine
reduce
ShakewithNall to
Tint conc Hcl
Thenwithcooling
waterbath
heaton a boiling
complete the reduction
x
Base pyridinesHsn Yagatl
Benzoylchloride
lessreactive
speedupreaction
OH acyl
chloride
ethanol chloride on
Na
Ha CHIEF ftbÉd dilutenitrigacid
t
ester
gBrafranset 70 Nos
test
forphenols Fed
OH it producesapurplesolution
Br Br
to
c
c.si moreoften
f
Colourlesssolution
whiteprecipitate
NIN NHI
Br T
t ftp.litgqgene.coaplingreat it
, benzenediazonium chloride
gg
ftNatal NENCT
fidN alkalinesolution
x
diazotisation
excess nitrousacid at ie
it below lot it would bestable
OH unilatalititaticamine which
yellowppt if haveNaoly
ng need include thosetoo
so Nacl H2O very
unstableandbecome alcohol immediately
brijeg
NH d
dye Nth
acid base
NaOH liberatethe
phenylamine
phenylamine
Reduction f
Collect by steamdistillation
agent in water
thesolubility of
Reducing phenylamine
reduce
ShakewithNall to
Tint conc Hcl
Thenwithcooling
waterbath
heaton a boiling
complete the reduction