Moles (Gizmo) questions and answers
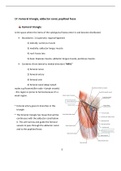
Moles (Gizmo) questions and answers The jars below, from L to R, each contain exactly one mole of carbon, sulfur, and aluminum, respectively. Which jar contains the most atoms? D. Each jar contains the same number of atoms If each jar below contains exactly one mole of each element, which jar contains the greatest mass? B. The jar of sulfur Note the molecular mass of copper (I) oxide molecule in the image below. What is the mass of 8.250 moles of Cu2O? D. 1181 g What is the mass of 8.12 x 10^23 molecules of CO2 gas? (Atomic mass of carbon = 12.011 u; oxygen = 15.999 u) D. 59.3 g Sulfuric acid (H2SO4) has a molar mass of 98.1 g/mol. How many oxygen atoms are found in 75.0 g of H2SO4? B. 1.84 x 10^24
Written for
- Institution
- Gizmos
- Course
- Gizmos
Document information
- Uploaded on
- February 15, 2023
- Number of pages
- 1
- Written in
- 2022/2023
- Type
- Exam (elaborations)
- Contains
- Questions & answers
Subjects
- the jars below
- from l to r
- sulfur
- and aluminum
-
moles gizmo questions and answers
-
each contain exactly one mole of carbon
-
respectively which jar contains the most atoms d each jar contains th
Also available in package deal