MODULE 2: NEURODEGENERATIVE DISORDERS
Lecture 6: general mechanisms in neuro-degeneration
Neurodegeneration: loss of brain cells and/or loss of brain function
Protein aggregates: protein misfolds (or is
unfolded) and therefore clump together, they
form aggregates.
- loss of function
- gain of toxic function
➔ therefore: protein quality control
synthesis→non-functional
conformation→functional conformation
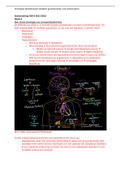
Figure 8, specific disease examples in this course (Alzheimer, frontotemporal
protein synthesis: dementia, Huntington, Parkinson, Lewy body dementia, prion disease,
amyotrophic lateral sclerosis
- in the cytosol (not specifically in an
organel)
- endoplasmic reticulum (ER)
o secreted proteins
o TM proteins (transmembrane)
o Organel targeted proteins
Chaperones (heat-shock proteins, HSP)
- Recognition/refolding. They bind to unfolded, non-functional proteins and give it a change to
fold a second time. Non-functional conformation → functional conformation. It prevents
aggregation.
- Chaperone facilitate protein folding and refolding by keeping proteins in ‘a folding-
competent state’
- ATP dependent: Hsp90, HspP70!, Hsp60
- ATP independent: small Hsp
The Hsp70 cycle (figure 9):
- Binding and release of substrate
(unfolded protein) is dependent on :
o Co-chaperone Figure 9
o ATP
Degradation of unfolded proteins: If the chaperones fail.
- Ubiquitin-proteasome system (UPS). Multi enzyme complex
o Ubiquitination of target protein. And recognition of poly Ub tag
o Catalytic chamber in the proteosome.
▪ Proteasome inhibition induces accumulation of polyUb proteins.
▪ Reduced proteasome activity in neurodegenerative disease (AD, HD, PD)
▪ Ubiquitin positive inclusions in Alzheimer disease brain.
- Autophagy.
37
, o Formation of autophagosome: Double membrane is wrapped around the substrate
o Autolysosome :The autophagosome fuses with the lysosome.
▪ Autophagy is important for quality control
- So the proteolytic pathways (pathway that breaks down a
protein):
o Proteasome: degrades only monomeric proteins
o Autophagy: can degrade larger structures: organelles,
aggregate.
Aggregate formation: oligomers → fibrils → inclusion body
Neurodegenerative diseases aetiology:
- Sporadic
Figure 10
- Acquired
- Genetic
o Typically sporadic / acquired and genetic variant of same disease
Genetic causes of neurodegeneration:
- Mutation found in:
o Genes that encoded the main component of the aggregate:
▪ Gene dosage
▪ Missense, deletion
o Genes that encode other proteins: clue to mechanism
Genetic only: huntington’s disease:
- Genetic
- Aggregates of huntingtin protein
- Basal ganglia (movement phenotype)
- Chorea (typical type of movement)
Aggregates are caused by a repeat (CAG), which results in more glutamine. The protein starts to
form aggregates. The repeat length drives aggregation of Huntingtin.
- Wild-type htt <35Q
- Mutant Htt >35 Q
Neurodegenerative disease:
- Common:
o Protein misfolding and aggregation
o Inadequate protein quality control
o Sporadic and genetic (except HD)
o Oligomers/fibrils
o Stress responses
o Neuro-inflammation
- Different:
o Protein in aggregates
o Subcellular compartment and brain region affected
o Gain of toxic function/ loss of function
38
, Fibril formation and disease are connected, but not quantitatively
- Many deposits but little or no disease
- Little or no deposits but severe disease
➔ Aggregation state: No correlation with disease severity
Inclusion bodies, toxic or protective?
Mitotic clearance of aggregates. (Neurons don’t divide), figure 11
Neurodegenerative disease: protein aggregation → synaptic dysfunction → neuronal loss Figure 11
- Protein aggregates disrupt neuronal function
- Oligomeric aggregates can directly affect synapses
Protective responses of the brain:
- Cellular responses to unfolded protein stress
- Neuroinflammation
Responses to misfolded protein stress:
- Activated in response to accumulation of misfolded proteins
- Dependent on localization of misfolding / aggregation
- Transient response intended to restore protein folding homeostasis Figure 12
Stress responses:
- Misfolding in the cytosol: Heat-shock response (figure 12)
- misfolding in the ER: the unfolded protein response (UPR) (figure 13)
o UPR: accumulation of misfolded proteins → BIP titrated from
sensors, sensors activated, UPR induced.
▪ UPR activation changes transcriptome and proteome
Stress responses:
- Dependent on subcellular localization of aggregate
Figure 13, PERK, ATF6, IRE1
- Chaperone bound to stress transducers
- Accumulation of misfolded proteins titrates chaperones from the stress transducers
- Release from chaperone activates the stress transducer
- Activation of transcriptional/translational program
Definition of inflammation: inflammation is a localized non-specific biological response to defend,
repair, or alter bodily components. It accompanies infections, trauma, neoplasm, physiological
stresses, degenerative changes, and certain metabolic disorders. It is triggered by tissue injury to
destroy, dilute, or wall off (sequester) stimulating agents and injured structures. Elimination of
neutralization of devitalized or intruding elements and repair of the defect are included. The process
is similar in all tissues, but is modified by the cause.
Neuroinflammation:
- Astrocytes activation <-> microglial activation. Pro-inflammatory elements activates more
glial activation
- Reactive astrogliosis in many neurological diseases.
o GFAP upregulation
39
Lecture 6: general mechanisms in neuro-degeneration
Neurodegeneration: loss of brain cells and/or loss of brain function
Protein aggregates: protein misfolds (or is
unfolded) and therefore clump together, they
form aggregates.
- loss of function
- gain of toxic function
➔ therefore: protein quality control
synthesis→non-functional
conformation→functional conformation
Figure 8, specific disease examples in this course (Alzheimer, frontotemporal
protein synthesis: dementia, Huntington, Parkinson, Lewy body dementia, prion disease,
amyotrophic lateral sclerosis
- in the cytosol (not specifically in an
organel)
- endoplasmic reticulum (ER)
o secreted proteins
o TM proteins (transmembrane)
o Organel targeted proteins
Chaperones (heat-shock proteins, HSP)
- Recognition/refolding. They bind to unfolded, non-functional proteins and give it a change to
fold a second time. Non-functional conformation → functional conformation. It prevents
aggregation.
- Chaperone facilitate protein folding and refolding by keeping proteins in ‘a folding-
competent state’
- ATP dependent: Hsp90, HspP70!, Hsp60
- ATP independent: small Hsp
The Hsp70 cycle (figure 9):
- Binding and release of substrate
(unfolded protein) is dependent on :
o Co-chaperone Figure 9
o ATP
Degradation of unfolded proteins: If the chaperones fail.
- Ubiquitin-proteasome system (UPS). Multi enzyme complex
o Ubiquitination of target protein. And recognition of poly Ub tag
o Catalytic chamber in the proteosome.
▪ Proteasome inhibition induces accumulation of polyUb proteins.
▪ Reduced proteasome activity in neurodegenerative disease (AD, HD, PD)
▪ Ubiquitin positive inclusions in Alzheimer disease brain.
- Autophagy.
37
, o Formation of autophagosome: Double membrane is wrapped around the substrate
o Autolysosome :The autophagosome fuses with the lysosome.
▪ Autophagy is important for quality control
- So the proteolytic pathways (pathway that breaks down a
protein):
o Proteasome: degrades only monomeric proteins
o Autophagy: can degrade larger structures: organelles,
aggregate.
Aggregate formation: oligomers → fibrils → inclusion body
Neurodegenerative diseases aetiology:
- Sporadic
Figure 10
- Acquired
- Genetic
o Typically sporadic / acquired and genetic variant of same disease
Genetic causes of neurodegeneration:
- Mutation found in:
o Genes that encoded the main component of the aggregate:
▪ Gene dosage
▪ Missense, deletion
o Genes that encode other proteins: clue to mechanism
Genetic only: huntington’s disease:
- Genetic
- Aggregates of huntingtin protein
- Basal ganglia (movement phenotype)
- Chorea (typical type of movement)
Aggregates are caused by a repeat (CAG), which results in more glutamine. The protein starts to
form aggregates. The repeat length drives aggregation of Huntingtin.
- Wild-type htt <35Q
- Mutant Htt >35 Q
Neurodegenerative disease:
- Common:
o Protein misfolding and aggregation
o Inadequate protein quality control
o Sporadic and genetic (except HD)
o Oligomers/fibrils
o Stress responses
o Neuro-inflammation
- Different:
o Protein in aggregates
o Subcellular compartment and brain region affected
o Gain of toxic function/ loss of function
38
, Fibril formation and disease are connected, but not quantitatively
- Many deposits but little or no disease
- Little or no deposits but severe disease
➔ Aggregation state: No correlation with disease severity
Inclusion bodies, toxic or protective?
Mitotic clearance of aggregates. (Neurons don’t divide), figure 11
Neurodegenerative disease: protein aggregation → synaptic dysfunction → neuronal loss Figure 11
- Protein aggregates disrupt neuronal function
- Oligomeric aggregates can directly affect synapses
Protective responses of the brain:
- Cellular responses to unfolded protein stress
- Neuroinflammation
Responses to misfolded protein stress:
- Activated in response to accumulation of misfolded proteins
- Dependent on localization of misfolding / aggregation
- Transient response intended to restore protein folding homeostasis Figure 12
Stress responses:
- Misfolding in the cytosol: Heat-shock response (figure 12)
- misfolding in the ER: the unfolded protein response (UPR) (figure 13)
o UPR: accumulation of misfolded proteins → BIP titrated from
sensors, sensors activated, UPR induced.
▪ UPR activation changes transcriptome and proteome
Stress responses:
- Dependent on subcellular localization of aggregate
Figure 13, PERK, ATF6, IRE1
- Chaperone bound to stress transducers
- Accumulation of misfolded proteins titrates chaperones from the stress transducers
- Release from chaperone activates the stress transducer
- Activation of transcriptional/translational program
Definition of inflammation: inflammation is a localized non-specific biological response to defend,
repair, or alter bodily components. It accompanies infections, trauma, neoplasm, physiological
stresses, degenerative changes, and certain metabolic disorders. It is triggered by tissue injury to
destroy, dilute, or wall off (sequester) stimulating agents and injured structures. Elimination of
neutralization of devitalized or intruding elements and repair of the defect are included. The process
is similar in all tissues, but is modified by the cause.
Neuroinflammation:
- Astrocytes activation <-> microglial activation. Pro-inflammatory elements activates more
glial activation
- Reactive astrogliosis in many neurological diseases.
o GFAP upregulation
39