Natural Science
GRADE 9
1 Grade 9 Natural Sciences I © 2026 Noted™ www.notedsummaries.co.za
, Science
number of protons
ELEMENTS
Atomic number
(and = electrons if the atom is
Mass number
neutral).
= protons + neutrons
Symbol
Atom = smallest particle of an ** 1 Uppercase + lowercase
(e.g. Na, not NA)
element
Molecules
2 or more atoms that bond
MOLECULES
together.
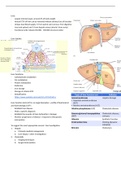
NEUKLEUS ORBITAL ELEMENTMOLECULE COMPOUND MOLECULE
ELECTRONS
NEGATIVELY charged particles
Electron (e⁻) = negative,aroundnucleus
Diatomic elements
Diatomic = made up of 2 of
different atoms (e.g. H2O, CO2)
WITH fixed ratio of atoms (e.g.
the same atoms in a molecule. water = 2 H : 1 O)
PROTONS
POSITIVELYcharged particles
NEUTRONS
Neutron (n⁰) = neutral, in nucleus
H2, O2, F2, Br2, I2, N2, Cl2 What holds the atoms (molecules)
together?
Chemical bonding= forces that hold atoms
2 Proton (p⁺) = positive,in nucleus Grade 9 Natural Sciences I © 2026 Noted™ www.notedsummaries.co.za together in a molecule/compound.
, ELEMENTS Dmitri Mendeleev
Russian scientist → 1869
Element= pure substance→ cannot be broken down. Arrangeelements according toatomic mass
→ Name Atomic number Elements =repeating patterns→ "periodic"
→ Symbol
→ Atomic number (electrons) 3 MAIN GROUPS ON THE TABLE:
Relative Periods = rows (left to right)→ 7
→ Unique position on Table atomic mass Groups = columns (top to bottom) → 18
Symbol Elements in samegroup = similar properties
** 1 Uppercase + lowercase Group Where? Characteristics (short)
(e.g. Na, not NA) Metals Left & middle ∗ Shiny
GROUPS ∗ Malleable / ductile
∗ Good conductors
∗ Mostly solid substances
1 Non-metals Right ∗ solid/liquid/gas
PERIODS
2 ∗ Poor conductors (mostly)
3 Semi-metals Dividing line in Mixed properties
4 between
5 ⚠️ Hydrogen (H) non = -metal(but placed top left) = exceptional
6 element.
IMPORTANT:
NOBLE GASES→ Group 18
Atomic number increases
Very stable(react minimally )
Examples: He, Ne, Ar from left to right in a period
3 Grade 9 Natural Sciences I © 2026 Noted™ www.notedsummaries.co.za
, FIRST 20 ELEMENTS
Atomic# Name Symbol Everyday Use
1️⃣ Hydrogen H Rocket fuel • Watering
2️⃣ Helium He Balloons • Refrigerant in MRIs
3️⃣ Lithium Li Cell Phone Batteries • Anti-
depressants 💊
4️⃣ Beryllium Be Aerospace Alloys• X-ray
5️⃣ Boron B Washing Powder• Sports Equipment
6️⃣ Carbon C Pencils • Diamante💎
7️⃣ Nitrogen N Fertilizer 🌾 • Air (78%)🌬️
8️⃣ Oxygen O Breathing🫁 • Fire 🔥• Water💧
9️⃣ Fluorescent F Toothpaste• Iron processing
🔟 Neon Ne Neon lights
1️⃣1️⃣ Sodium Na Table salt (NaCl)🧂
1️⃣2️⃣ Magnesium Mg Fireworks• Muscles
1️⃣3️⃣ Aluminum Al Cans • Foil Strips• Airplanes
1️⃣4️⃣ Silicon Si Computer Chips• Solar Panels
1️⃣5️⃣ Phosphorus P Matches • Fertilizer
1️⃣6️⃣ Sulfur S Sulfuric acid • Rubbers• Explosions
1️⃣7️⃣ Chlorine Cl Pool Cleaning• Bleach🧴
1️⃣8️⃣ Argon Ar Bulbs
1️⃣9️⃣ Potassium K Nerve function
2️⃣0️⃣ Calcium Ca Bones🦴 • Milk🥛 • Shells🐚
4 Grade 9 Natural Sciences I © 2026 Noted™ www.notedsummaries.co.za