answers 2025\2026 A+ Grade
Given a gram of a 1:1 mixture benzoic acid and cholesterol, describe how you would separate the two
compound.
- correct answer 1. In a separatory funnel, dissolving each in ether. Add a base.
Organic layer will contain the ether
Aqueous layer will contain the acid
Benzamine and cholesterol are dissolved in ether. How would you separate them?
- correct answer Place into a separatory funnel and add a strong acid
Organic layer will contain ether
Aqueous layer will contain the acid
a) What are the properties of a good recrystallization solvent.
b) When would you use a mixed recrystallizing solvent system rather than an single solvent system.
- correct answer 1. a) @ room temp, solvent should not dissolve
@ boiling temp, solid should completely dissolve
Should have boiling point lower than melting point of solid that is being purified
Solvent should not react with solid being purified
b) When a single solvent wont work
Why use a Buchner funnel to collect crystals rather than gravity filtration apparatus?
- correct answer Vacuum filtration is more efficient when the solid is the desired material (i.e. removing
the solvent from the crystals)
A compound dissolves readily in ether at room temperature and not at all in water are room
temperature . Why is ether and water still not able to be used for a mix solvent recrystallization?
- correct answer The compound must be soluble in water at room temp as well
, Given a list of reagents, give the products of the reaction ( covered in the lab
a) dehydration
b) bromination
- correct answer a) 1-methylcycohexene, 3-methyl-1-cyclohexene, 4-methy-1-lcyclohexene,
methylenecyclohexene, ethylidenecyclopentene
b) 1,2-dibromo-1,2-diphenylethane
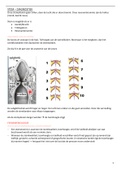
Draw the mechanism for the dehydration and bromination reactions. Use correct arrow, and show all
intermediates.
- correct answer Refer to Photos
After the reaction, a product was distilled. The distillate was placed in a separatory funnel and washed
with NaCl (sat) . Why was the distillate washed with brine?
- correct answer The brine pulls the remaining water from the organic layer to the aqueous layer.
Given the density , molecular weight and the volume or mass of reagents:
a) calculate the mols of reagent, equivalence and determine the limiting reagent.
b) given the product yield -calculated the % yield.
- correct answer a) use starting material to find common product and use the lowest amount created as
limiting reagent
What is a catalyst
- correct answer Substance that increases the rate of a reaction without being used up by lowering
activation energy (Ea).
What is indicated by melting point range.
- correct answer Point at where it's beginning to liquify and indicates identity and purity
a) What is indicated by melting point depression.
b ) Given a number of standard compound that have the same melting point as your product - What
techniques could you use to give PROOF of the identity of your product?
- correct answer a) The purity of the compound. Impurities cause depression as there it interference
with crystal lattice energies