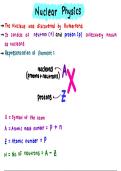Nuclear Physics
The Nucleus was discovered by Rutherford .
It consist of Neutron (1) and proton (p) collectively known
as nucleons
.
Representation of Element :
nucleons
protons + heutrons
:
A
Protons z
-
symbol of the atom
- - Atomic mass number
-
P M
-
Atomic number
-
P
-
No .
Of Meutrons --
, Types of Nuclei :
Same z =
isotopes S
2
S
3
· 3 e , e
3 3
Same A-isobars ·
C
same X-isotones
3
·
"e
Q
. The nuclei sC" and IN'4 can be described as
(a) isotones (b) isobars
() isotopes of Carbon (d) isotopes of nitrogen
,Atomic masses :
liogram (
Atomic mass of 1sCatom
Mass Unit (4) -
12
u -
.
6605402/10-27kg
e This unit is used to measure masses in terms of their
: energy
u -
93 . 5/e
,
1
⑨ H ec
9 09383 x 10-31 0 00055 0 51
My
. . .
Mp . 672623 x 10-27 1 . 007276 938 . 28
Mn 1 . 674927 x 10-27 1 . 008665 939 57 .
, Size of Nucleus :
size of a nucleus depends on number of nucleons present in it
Nuclear Radius :
R-Rol where , Ro-1 . 2 x 10-15 m = 1 2
. fm
'13
R L ↓
Nuclear volume :
3is
G
The Nucleus was discovered by Rutherford .
It consist of Neutron (1) and proton (p) collectively known
as nucleons
.
Representation of Element :
nucleons
protons + heutrons
:
A
Protons z
-
symbol of the atom
- - Atomic mass number
-
P M
-
Atomic number
-
P
-
No .
Of Meutrons --
, Types of Nuclei :
Same z =
isotopes S
2
S
3
· 3 e , e
3 3
Same A-isobars ·
C
same X-isotones
3
·
"e
Q
. The nuclei sC" and IN'4 can be described as
(a) isotones (b) isobars
() isotopes of Carbon (d) isotopes of nitrogen
,Atomic masses :
liogram (
Atomic mass of 1sCatom
Mass Unit (4) -
12
u -
.
6605402/10-27kg
e This unit is used to measure masses in terms of their
: energy
u -
93 . 5/e
,
1
⑨ H ec
9 09383 x 10-31 0 00055 0 51
My
. . .
Mp . 672623 x 10-27 1 . 007276 938 . 28
Mn 1 . 674927 x 10-27 1 . 008665 939 57 .
, Size of Nucleus :
size of a nucleus depends on number of nucleons present in it
Nuclear Radius :
R-Rol where , Ro-1 . 2 x 10-15 m = 1 2
. fm
'13
R L ↓
Nuclear volume :
3is
G