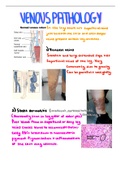
Set (2025), Exams of Organic Chemistry.
Question. Which of the following aqueous solutions would have the highest boiling point?
A) 1.0 m NaNO3
B) 1.0 m HF
C) 1.0 m C₆H₁₂O₆
D) 1.0 m CaCl2 -Correct Ans✓✓ The dissolved solute will raise the boiling point. The more "things" that are
dissolved (ions or molecules), the higher the boiling point will be. The CaCl₂ will break up into one Ca²⁺ ion and
two Cl⁻ ions and there will be a concentration of 3.0 m of "things" in solution. The other choices will have fewer
"things" in solution and will raise the boiling point less.
Question. Which one of the following aqueous solutions would have the highest freezing point?
A) 1.0 m NaNO3
B) 2.0 m CH3OH
C) 1.0 m C₆H₁₂O₆
D) 1.0 m CaCl2 -Correct Ans✓✓ The dissolved solute will lower the freezing point. The more "things" that are
dissolved (ions or molecules), the lower the freezing point will be. Since C₆H₁₂O₆ is a molecule, it will not break
apart and there will only be a concentration of 1.0 m of molecules in solution. It will lower the freezing point
less than all the other choices. There will be fewer "things" in solution and this will lower the freezing point less
than the other choices.
Question. isoelectronic ions -Correct Ans✓✓ ions containing the same number of electrons
Question. Which of the following bonds is the most polar? -Correct Ans✓✓ Elements that are located far away
from each other on the periodic table have larger electronegativity differences and therefore make more polar
bonds. Of all the element pairs listed, Rb and Br are the farthest apart.
1|Page
,Question. Three blocks of the same shape and size are placed in front of you. Block A has a mass of 3.0 g,
block B is 5.0 g, and block C is 10.0 g. Which has the higher density? -Correct Ans✓✓ Density is the ratio of
mass to volume. Since the volume is constant between all the blocks, then the block with the highest mass will
have the highest density.
Question. Density equation -Correct Ans✓✓ mass/volume
dRT = PM
Question. The solubility of a gas in a liquid generally
A) increases with increasing temperature.
B) increases with decreasing temperature.
C) increases with increasing pressure.
D) both (a) and (c).
E) both (b) and (c). -Correct Ans✓✓ E) both (b) and (c)
Question. Which of the following properties, in general, increase as we move left to right across a period in the
periodic table?
1. atomic radius
2. ionization energy
3. metallic character
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2 only
E) 2 and 3 only -Correct Ans✓✓ B) 2 only
2|Page
,Question. All the following statements concerning a sample of oxygen gas at 1.00 atm pressure are true
EXCEPT
A) the molecules are in constant rapid random motion.
B) the pressure exerted by gaseous oxygen is due to the impact of the molecules with the walls of the container.
C) the average kinetic energy of the gaseous oxygen is inversely proportional to the absolute temperature of the
gas.
D) collisions between the gaseous molecules are elastic.
E) the volume occupied by the oxygen molecules is negligible compared with the size of the container. -Correct
Ans✓✓ C) the average kinetic energy of the gaseous oxygen is inversely proportional to the absolute
temperature of the gas.
Question. A bond in which an electron pair is unequally shared by two atoms is
A) ionic.
B) polar covalent.
C) nonpolar covalent.
D) coordinate covalent.
E) dipolar. -Correct Ans✓✓ B) polar covalent.
Question. Which of the following statements is (are) true?
1. An excited atom can return to a higher energy level by emitting light energy.
2. An atom can be excited to a higher energy level by absorption of light energy.
3. The frequency and wavelength of light are inversely proportional.
A) 1 only
B) 2 only
C) 1 and 3 only
D) 2 and 3 only
3|Page
, E) 1, 2, and 3 -Correct Ans✓✓ D) 2 and 3 only
Question. Solvent -Correct Ans✓✓ A liquid substance capable of dissolving other substances
Question. Solute -Correct Ans✓✓ A substance that is dissolved in a solution.
Question. Solution -Correct Ans✓✓ A homogeneous mixture that forms when one substance dissolves another.
Question. The measure of the attraction that an atom has for the electrons in a chemical bond is called
A) electron affinity.
B) ionization energy.
C) electronegativity.
D) hybridization.
E) London forces. -Correct Ans✓✓ C) electronegativity.
Question. Avogadro's Law -Correct Ans✓✓ equal volumes of gases at the same temperature and pressure
contain equal numbers of molecules
Question. Boyle's Law -Correct Ans✓✓ Boyle's Law states that at constant temperature and moles, pressure
and volume are inversely proportional.
Mathematically this is written as: PV = PV
Question. Gay-Lussac's Law -Correct Ans✓✓ the pressure of a gas is directly proportional to the Kelvin
temperature if the volume is constant
4|Page