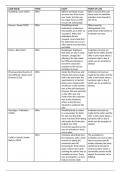
Chemical Equilibria
Complete reactions
o Reaction continues until one of the reactants is completely used up and the reaction
stops
Reversible reaction
o Reaction does not go to completion and is reversible
Dynamic equilibrium
o Rate of forward reaction = rate of backward reaction
o Concentrations of reactants and products remain constant
Changing Conditions of a Reaction in Equilibria
It is possible to change the proportions of reactants to products in an equilibrium mixture by
changing the conditions
An increased proportion of products will lead to the equilibrium shifting to the right (forward
direction)
A increased proportion of reactants will lead to the equilibrium shifting to the left (backward
direction)
Le Chateliers principle – A system at equilibrium will react to oppose any change imposed on it
Concentration
Increasing concentration of reactant – shifts to right
Decreasing concentration of reactant – shifts to left
Increasing concentration of product – shifts to left
Decreasing concentration of product – shifts to right
Pressure
Changing the total pressure will only change the position of equilibrium if there are different
numbers of molecules on either side for the equation as the concentration of reactants and
products is changed by the same amount
Increasing pressure – shifts to side with fewer moles of gas to reduce pressure – yield of side
with more moles decreases
Decreasing pressure – shifts to side with more moles of gas to increase pressure – yied of side
with more moles increases
Same amount of moles on both sides of equation – pressure has no effect on equilibrium
position but does affect rate
Temperature
In a reversible reaction, if the forward reaction is exothermic then the backward reaction is
endothermic
Temperature increase – equilibrium position shifts toward endothermic direction to reduce
temperature, yield of exothermic reaction decreases
Complete reactions
o Reaction continues until one of the reactants is completely used up and the reaction
stops
Reversible reaction
o Reaction does not go to completion and is reversible
Dynamic equilibrium
o Rate of forward reaction = rate of backward reaction
o Concentrations of reactants and products remain constant
Changing Conditions of a Reaction in Equilibria
It is possible to change the proportions of reactants to products in an equilibrium mixture by
changing the conditions
An increased proportion of products will lead to the equilibrium shifting to the right (forward
direction)
A increased proportion of reactants will lead to the equilibrium shifting to the left (backward
direction)
Le Chateliers principle – A system at equilibrium will react to oppose any change imposed on it
Concentration
Increasing concentration of reactant – shifts to right
Decreasing concentration of reactant – shifts to left
Increasing concentration of product – shifts to left
Decreasing concentration of product – shifts to right
Pressure
Changing the total pressure will only change the position of equilibrium if there are different
numbers of molecules on either side for the equation as the concentration of reactants and
products is changed by the same amount
Increasing pressure – shifts to side with fewer moles of gas to reduce pressure – yield of side
with more moles decreases
Decreasing pressure – shifts to side with more moles of gas to increase pressure – yied of side
with more moles increases
Same amount of moles on both sides of equation – pressure has no effect on equilibrium
position but does affect rate
Temperature
In a reversible reaction, if the forward reaction is exothermic then the backward reaction is
endothermic
Temperature increase – equilibrium position shifts toward endothermic direction to reduce
temperature, yield of exothermic reaction decreases