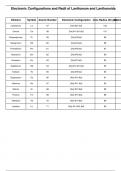
Metal Ion Color in Aqueous Solution
Sc3+ Colorless
Ti3+ Purple
Ti4+ Colorless
V2+ Violet
V3+ Green
VO2+ Blue
VO2+ Yellow
Cr3+ Green or Violet
Mn2+ Pale Pink
MnO4- Purple
Fe2+ Pale Green
Fe3+ Yellow-Brown
Co2+ Pink
Co3+ Dark Brown
Ni2+ Green
Cu2+ Blue
Zn2+ Colorless