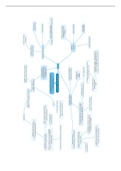
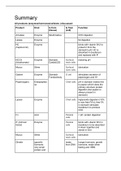
Pcc-1
Lecture 1
Chapter 9:
Strong acid: HA + H2O H3O+ + A-
Weak acid: HA + H2O ⇄ H3O+ + A-
Ka=¿ ¿
pKa = -log(Ka)
Mol/L HA H2O H3O+ A-
I C 0 0
C -x +x +x
E c-x x x
Ka=¿ ¿
X=[H3O+] pH
2 H2O ⇄ H3O+ + OH-
Kw=[H3O+] [OH-]
[H3O+] = √(Kw)
pKw=14
pH+pOH=14
pKa+pKb=14
Chapter 10 Acids and bases
An acid is a proton donor
A base is a proton acceptor
HA(aq) + H2O(l) ⇄ H3O+(aq) + A-(aq)
B(aq) + H2O(l) ⇄ BH+(aq) +OH-(aq)
A- is the conjugate base of the acid HA
BH+ is the conjugate acid of the base B
Amphoteric: can act both as an acid as a base.
Auto-ionization: in absence of other acids and bases still reacting with itself.
Kb= [ HA ] ¿ ¿
KaKb=¿ ¿
KaKb = Kw or pKa + pKb = pKw
, Chapter 11 Buffer solutions
Buffer: contains both HA and its conjugated base A-
Ka=x ¿ ¿
¿
−log ¿
pH= pKa+log ¿ ¿
If [HA]=[A-] then pH=pKa
-
If [HA]<[A ] then pH>pKa
If [HA]>[A-] then pH<pKa
Buffercapacity: ß
1
∗∆ x
v
ß=
¿ ∆ pH∨¿ ¿
v = volume
∆x = number of moles acid or base added
|∆pH| = absolute change of pH
Chapter 12 Adsorption
The species that is adsorbed is the adsorbate. The matter onto which the adsorption takes
place is called the adsorbent.
The adsorbed amount Γ is in mol/m2
x
Γ=
A
x = moles of adsorbate
A = area
x
X=
m
m = mass of adsorbent
The total area that is available for adsorption per gram of adsorbent is called the specific
surface area Asp
A
Asp=
m
x=ΓAsp
x Γ
⦵= =
xmax Γ max
⦵
= relative adsorption
Adsorption isotherm: relation adsorbed amount (ΓA of x) and its equilibrium concentration.
H2O(ads) + A(aq) ⇄ H2O(l) + A(ads)
Water and A same size and surface is completely covered:
Γmax=Γ H 2 O + Γ A
Lecture 1
Chapter 9:
Strong acid: HA + H2O H3O+ + A-
Weak acid: HA + H2O ⇄ H3O+ + A-
Ka=¿ ¿
pKa = -log(Ka)
Mol/L HA H2O H3O+ A-
I C 0 0
C -x +x +x
E c-x x x
Ka=¿ ¿
X=[H3O+] pH
2 H2O ⇄ H3O+ + OH-
Kw=[H3O+] [OH-]
[H3O+] = √(Kw)
pKw=14
pH+pOH=14
pKa+pKb=14
Chapter 10 Acids and bases
An acid is a proton donor
A base is a proton acceptor
HA(aq) + H2O(l) ⇄ H3O+(aq) + A-(aq)
B(aq) + H2O(l) ⇄ BH+(aq) +OH-(aq)
A- is the conjugate base of the acid HA
BH+ is the conjugate acid of the base B
Amphoteric: can act both as an acid as a base.
Auto-ionization: in absence of other acids and bases still reacting with itself.
Kb= [ HA ] ¿ ¿
KaKb=¿ ¿
KaKb = Kw or pKa + pKb = pKw
, Chapter 11 Buffer solutions
Buffer: contains both HA and its conjugated base A-
Ka=x ¿ ¿
¿
−log ¿
pH= pKa+log ¿ ¿
If [HA]=[A-] then pH=pKa
-
If [HA]<[A ] then pH>pKa
If [HA]>[A-] then pH<pKa
Buffercapacity: ß
1
∗∆ x
v
ß=
¿ ∆ pH∨¿ ¿
v = volume
∆x = number of moles acid or base added
|∆pH| = absolute change of pH
Chapter 12 Adsorption
The species that is adsorbed is the adsorbate. The matter onto which the adsorption takes
place is called the adsorbent.
The adsorbed amount Γ is in mol/m2
x
Γ=
A
x = moles of adsorbate
A = area
x
X=
m
m = mass of adsorbent
The total area that is available for adsorption per gram of adsorbent is called the specific
surface area Asp
A
Asp=
m
x=ΓAsp
x Γ
⦵= =
xmax Γ max
⦵
= relative adsorption
Adsorption isotherm: relation adsorbed amount (ΓA of x) and its equilibrium concentration.
H2O(ads) + A(aq) ⇄ H2O(l) + A(ads)
Water and A same size and surface is completely covered:
Γmax=Γ H 2 O + Γ A