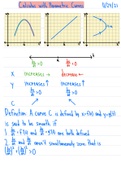
Particle Physics
Atoms, Radiation, Nucleii
Alpha particle scattering experiment
• The figure shows the apparatus used in the alpha-scattering experiment
① The alpha-particle source was encased in metal with a small aperture allowing a fine
beam of alpha-particles to emerge
② Air in the apparatus was sucked out to leave a vacuum
→ Alpha particles / Alpha radiation are absorbed by a few centimeters of air
③ One reason for choosing gold is that it can be made into a very thin sheet/foil (few
atomic layers)
④ The alpha particles were detected when they struck a solid 'scintillating' material.
Each alpha particle gave a tiny flash of light and these were counted by the
experimenters
⑤ The detector could be moved round to detect alpha particles scattered through
different angles
, Graph of number of particles against angle
No of particles, N
I
1800
9100
g
o
1800 Angle /
scattering angle
Atoms, Radiation, Nucleii
Alpha particle scattering experiment
• The figure shows the apparatus used in the alpha-scattering experiment
① The alpha-particle source was encased in metal with a small aperture allowing a fine
beam of alpha-particles to emerge
② Air in the apparatus was sucked out to leave a vacuum
→ Alpha particles / Alpha radiation are absorbed by a few centimeters of air
③ One reason for choosing gold is that it can be made into a very thin sheet/foil (few
atomic layers)
④ The alpha particles were detected when they struck a solid 'scintillating' material.
Each alpha particle gave a tiny flash of light and these were counted by the
experimenters
⑤ The detector could be moved round to detect alpha particles scattered through
different angles
, Graph of number of particles against angle
No of particles, N
I
1800
9100
g
o
1800 Angle /
scattering angle