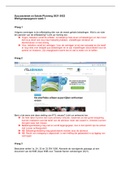
Gizmo Warm-up
The photoelectric effect occurs when tiny packets of light, called photons, knock electrons away from a
metal surface. Only photons with enough energy are able to dislodge electrons.
In the Photoelectric Effect Gizmo, check that the Wavelength is 500 nm, the Photon flux is 5 γ/ms, the
Voltage is 0.0 volts, and Potassium is selected. Click Flash the light to send photons of light (green arrows)
toward a metal plate encased in a vacuum tube.
1. The blue dots on the metal plate are electrons. What happens when the photons hit the electrons?
When the photons hit the electrons, half of the Electrons were knocked off of the potassium.
2. What happens when the electrons reach the light bulb?
When the electrons reach the light bulb, the light bulb lights up.
When electrons reach the light bulb they complete a circuit, causing the bulb to glow briefly.
Activity A: Get the Gizmo ready:
Wavelength and ● Check that the Voltage is 0.0 volts and Potassium is
flux selected.
Introduction: Through the centuries, many scientists have debated whether light is a wave or a stream of
tiny particles. In the 1800’s, most scientists agreed that phenomena such as refraction and diffraction
supported the “light as a wave” theory. However, Albert Einstein’s explanation of the photoelectric effect
showed that light can act like a stream of particles as well.
Question: What factors affect the ability of light to free electrons from a metal surface?
1. Observe: Click Flash the light with a variety of wavelength values. What do you notice?
I notice that the speed of the electron being knocked off gets slower as the wavelength increases and gets
faster as the wavelength decreases.
2. Observe: The photon flux is a measure of how bright the light is. It is equal to the number of
photons that are released in a given time. It is given as photons (γ) per millisecond (ms). Click Flash the
light with a variety of Photon flux values. What do you notice?
I notice that the Photon flux determines how many electrons get knocked off.
3. Form hypothesis: Answer the following questions based on what you have observed so far.
A. Which factor determines how many photons will strike the metal?
The factor that determines how many protons will strike the metal is how bright the original light is that is
going to bounce off of the metal.
Explain: The number of Photons are directly correlated to the brightness of the light and the more Photons
there are the more electrons will bounce off of the metal.
B. Which factor determines how much energy each photon has?
The factor that determines how much energy each photon has is wavelength.
Explain: The shorter the wavelength is, the faster the electrons get knocked off.
4. Investigate: Set the Photon flux to 1 γ/ms. Use the Gizmo to find the longest wavelength that will
dislodge an electron from the metal surface. What is this wavelength?
The longest wavelength that will dislodge an electron from the metal surface is 530nm.
5. Predict: Set the Wavelength to 540 nm. What do you think will happen if you flash the light with a
photon flux of 1 γ/ms? What if you flash the light with a flux of 10 γ/ms?
This study source was downloaded by 100000830772748 from CourseHero.com on 02-05-2022 01:53:36 GMT -06:00
https://www.coursehero.com/file/85811804/Aileen-Kang-Gizmo-Lab-Photoelectric-Effect-Studentdocx/