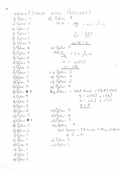Carbohydrates
Carbohydrates
Usually made up of carbon, hydrogen and oxygen
The general formula is CH2O, and this is repeated many times ()n.
There are three main types of carbohydrates:
Monosaccharides – simple sugars
Disaccharides – 2 monosaccharides
Polysaccharides – chains of many monosaccharides
Monosaccharides
Monosaccharides are very simple carbohydrates. They are the basic carbohydrate monomer. They are often used to
form larger, complex carbohydrates.
Trioses – 3 carbons: C3H6O3
Pentoses – 5 carbons: C5H10O5 - Pentose sugars are components of nucleic acids (ribose and deoxyribose) and
ATP
Hexoses – 6 carbons: C6H12O6 eg: glucose (alpha glucose and beta glucose), fructose. Hexose monosaccharides
are very important energy sources in living organisms
Glucose
Alpha-glucose
Alpha glucose is the basic subunit of starch and glycogen
Beta-glucose
Beta-glucose is the monomer of cellulose
, Test for Glucose
Clinistix is strips of cards containing an immobilised enzyme and indicator at one end which will change colour in
the presence of glucose.
Commonly used to test urine for glucose (diabetes)
They do not change colour in any other sugar
Colour change: Pink to Purple
Fructose
Hexose sugar
‘Fruit sugar.’ It is a monosaccharide found in many plants
Disaccharides
2 monosaccharides linked together by glycosidic bonds
When they join, water is released so this is called a condensation reaction
When they are spilt again, it is called a hydrolysis reaction.
This involves the addition of a water molecule
Hydrolysis is very important in the digestion of food
Eg:
o Maltose – when 2 alpha-glucose molecules join by condensation reaction. Maltose is formed when
starch is digested further to release the monomer glucose
o Sucrose – alpha-glucose and fructose condensation reaction. Sucrose is the form in which carbohydrates
are transported through the phloem in plants.
When maltose is formed a 1,4 glycosidic bond is formed. Between the 1 st carbon of the alpha glucose and the 4 th
carbon in the other alpha glucose molecule. Due to the orientation of the bond, the glucose molecules lie at a slight
angle when the bond is formed.
All disaccharides have the formula C 12H22O11 (H2O is taken out). Disaccharides are much more suitable for storage and
transport than monosaccharides
Sucrose is a disaccharide made up of glucose and fructose monomers. Sucrose is found in cane/sugar beet.
Reducing Sugars
Glucose, fructose, maltose and lactose are all reducing sugars
They all have carbonyl groups (C=O) which can be oxidised to carboxylic acids (-COOH)
Sucrose is not a reducing sugar as there are no reducing groups
Test for reducing sugars
Add an equal volume of Benedict’s reagent to the test solution and heat in at least 80 degrees in a water bath
Benedict’s is a blue solution containing copper sulphate
If a reducing sugar is present, the result will be an orange precipitate
If the solution remains blue, there is a non-reducing sugar present eg: sucrose
Blue (no reducing sugar) green yellow orange brick red precipitate
Test for non-reducing sugar eg: sucrose
Initially test some of the solution with Benedict’s to gain a negative result.
The rest of the sample is hydrolysed by heating with dilute hydrochloric acid in a water bath.
Once cool, neutralise by adding sodium hydrogen carbonate. Repeat Benedict’s and this a positive test with form
Carbohydrates
Usually made up of carbon, hydrogen and oxygen
The general formula is CH2O, and this is repeated many times ()n.
There are three main types of carbohydrates:
Monosaccharides – simple sugars
Disaccharides – 2 monosaccharides
Polysaccharides – chains of many monosaccharides
Monosaccharides
Monosaccharides are very simple carbohydrates. They are the basic carbohydrate monomer. They are often used to
form larger, complex carbohydrates.
Trioses – 3 carbons: C3H6O3
Pentoses – 5 carbons: C5H10O5 - Pentose sugars are components of nucleic acids (ribose and deoxyribose) and
ATP
Hexoses – 6 carbons: C6H12O6 eg: glucose (alpha glucose and beta glucose), fructose. Hexose monosaccharides
are very important energy sources in living organisms
Glucose
Alpha-glucose
Alpha glucose is the basic subunit of starch and glycogen
Beta-glucose
Beta-glucose is the monomer of cellulose
, Test for Glucose
Clinistix is strips of cards containing an immobilised enzyme and indicator at one end which will change colour in
the presence of glucose.
Commonly used to test urine for glucose (diabetes)
They do not change colour in any other sugar
Colour change: Pink to Purple
Fructose
Hexose sugar
‘Fruit sugar.’ It is a monosaccharide found in many plants
Disaccharides
2 monosaccharides linked together by glycosidic bonds
When they join, water is released so this is called a condensation reaction
When they are spilt again, it is called a hydrolysis reaction.
This involves the addition of a water molecule
Hydrolysis is very important in the digestion of food
Eg:
o Maltose – when 2 alpha-glucose molecules join by condensation reaction. Maltose is formed when
starch is digested further to release the monomer glucose
o Sucrose – alpha-glucose and fructose condensation reaction. Sucrose is the form in which carbohydrates
are transported through the phloem in plants.
When maltose is formed a 1,4 glycosidic bond is formed. Between the 1 st carbon of the alpha glucose and the 4 th
carbon in the other alpha glucose molecule. Due to the orientation of the bond, the glucose molecules lie at a slight
angle when the bond is formed.
All disaccharides have the formula C 12H22O11 (H2O is taken out). Disaccharides are much more suitable for storage and
transport than monosaccharides
Sucrose is a disaccharide made up of glucose and fructose monomers. Sucrose is found in cane/sugar beet.
Reducing Sugars
Glucose, fructose, maltose and lactose are all reducing sugars
They all have carbonyl groups (C=O) which can be oxidised to carboxylic acids (-COOH)
Sucrose is not a reducing sugar as there are no reducing groups
Test for reducing sugars
Add an equal volume of Benedict’s reagent to the test solution and heat in at least 80 degrees in a water bath
Benedict’s is a blue solution containing copper sulphate
If a reducing sugar is present, the result will be an orange precipitate
If the solution remains blue, there is a non-reducing sugar present eg: sucrose
Blue (no reducing sugar) green yellow orange brick red precipitate
Test for non-reducing sugar eg: sucrose
Initially test some of the solution with Benedict’s to gain a negative result.
The rest of the sample is hydrolysed by heating with dilute hydrochloric acid in a water bath.
Once cool, neutralise by adding sodium hydrogen carbonate. Repeat Benedict’s and this a positive test with form