Titration Practical:
Finding the concentration of sodium hydroxide (NaOH)
Introduction:
Titration4: A technique where a solution of known concentration is used to
determine the concentration of the unknown concentration.
Aims:
To determine the reacting volumes of solutions of an acid and alkali by
titration.
To determine the concentration of Sodium hydroxide in mol/dm3.
Calibrations:
Meaning: To adjust something such as measuring devices so that it can be used
in an accurate way and exact way possible.
What am I calibrating:
Pipette
Burettes
Weighing scale- accurate masses (calibration weight)
Using Accurate masses for calibration is more precise because they are never
touched and are handled with precaution of tweezers. This is more accurate
than a regular weighing scale because each scale has its unique weight, which
includes: 1g, 2g, 5g, 10g and 20g. It is also specific in terms of measurement
since it does not pick up extra mass. E.g.: When touched by hands the natural
oils from our fingertips will transfer onto the accurate masses which can lead to
inaccuracy.
Density of water= 1g/cm3
,We can measure this using our weighing scale because 1g is the density of water
and the weighing scale weighs every gram, so this would be another precise way
to measure.
The scales of South and City College Birmingham are calibrated by an external
organization, that is why I will not be doing it myself.
5Putting a waste bucket underneath the burette to catch water, so that the
burette is full.
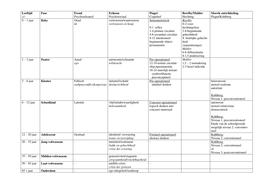
Equipment:
Burette
Bulb Pipette
Hydrochloric acid
25cm3 Sodium carbonate
Burette holder
250cm3 Conical flask x2
Methyl orange indicator
Weighing boat
Distilled water in a tube bottle
250ml Volumetric flask
Funnel
Weighing scale
Stirring rod
White tile
Marker
Boss
Waste bucket
Goggles
Beakers x2
Method:
Volumetric solution:
, 1. Calculate the mass of required substance needed to produce 250cm3 of a
0.100 mol dm-3 solution.
2. Use a weighing boat to weigh the mass. But before you weigh the mass,
weigh the weighing boat, and add it onto the mass total for accurate
measurements.
3. After transferring the mas of NaHCO3 (2.1g), reweigh the weighing boat in
case any residue is left in it. (Then not all mass was taken out which could
lead to inaccuracy within the experiment)
4. Transfer the mass into a 100ml beaker and add the least amount of
deionized water. Stir until dissolved.
5. Transfer dissolved solution to a volumetric flask (250ml). This can be
easily spillable so use a funnel to pour the mass in.
6. The volumetric flask has a line at the top (meniscus). Fill this line exactly
with deionized water carefully.
7. Rinse beaker and funnel in deionized (distilled) water and add it into the
volumetric flask (make sure)
8. Use a dropper to add drops of deionized water, doing this ever so
carefully so it does not overfill the line (at eyelevel is best). If so, please
repeat previous steps from 1 all over again.
9. Get the solution checked by a member of staff present, then put the lid in
the volumetric flask and invert it up and down 5-10 times, to ensure all
contents are mixed. Label it NaHC03.
Titration Part 13:
1. Pour approximately 100cm3 of the standard solution (NaHCO3) of known
concentration into a clean, dry beaker.
2. Fill the burette with the standard solution of known concentration (label
this HCl)
3. Pour approximately 100cm3 of the solution with unknown concentration
into a second beaker.
4. Make 250ml of a 0.1M solution of sodium hydrogen carbonate. (Get
calculations checked)
Finding the concentration of sodium hydroxide (NaOH)
Introduction:
Titration4: A technique where a solution of known concentration is used to
determine the concentration of the unknown concentration.
Aims:
To determine the reacting volumes of solutions of an acid and alkali by
titration.
To determine the concentration of Sodium hydroxide in mol/dm3.
Calibrations:
Meaning: To adjust something such as measuring devices so that it can be used
in an accurate way and exact way possible.
What am I calibrating:
Pipette
Burettes
Weighing scale- accurate masses (calibration weight)
Using Accurate masses for calibration is more precise because they are never
touched and are handled with precaution of tweezers. This is more accurate
than a regular weighing scale because each scale has its unique weight, which
includes: 1g, 2g, 5g, 10g and 20g. It is also specific in terms of measurement
since it does not pick up extra mass. E.g.: When touched by hands the natural
oils from our fingertips will transfer onto the accurate masses which can lead to
inaccuracy.
Density of water= 1g/cm3
,We can measure this using our weighing scale because 1g is the density of water
and the weighing scale weighs every gram, so this would be another precise way
to measure.
The scales of South and City College Birmingham are calibrated by an external
organization, that is why I will not be doing it myself.
5Putting a waste bucket underneath the burette to catch water, so that the
burette is full.
Equipment:
Burette
Bulb Pipette
Hydrochloric acid
25cm3 Sodium carbonate
Burette holder
250cm3 Conical flask x2
Methyl orange indicator
Weighing boat
Distilled water in a tube bottle
250ml Volumetric flask
Funnel
Weighing scale
Stirring rod
White tile
Marker
Boss
Waste bucket
Goggles
Beakers x2
Method:
Volumetric solution:
, 1. Calculate the mass of required substance needed to produce 250cm3 of a
0.100 mol dm-3 solution.
2. Use a weighing boat to weigh the mass. But before you weigh the mass,
weigh the weighing boat, and add it onto the mass total for accurate
measurements.
3. After transferring the mas of NaHCO3 (2.1g), reweigh the weighing boat in
case any residue is left in it. (Then not all mass was taken out which could
lead to inaccuracy within the experiment)
4. Transfer the mass into a 100ml beaker and add the least amount of
deionized water. Stir until dissolved.
5. Transfer dissolved solution to a volumetric flask (250ml). This can be
easily spillable so use a funnel to pour the mass in.
6. The volumetric flask has a line at the top (meniscus). Fill this line exactly
with deionized water carefully.
7. Rinse beaker and funnel in deionized (distilled) water and add it into the
volumetric flask (make sure)
8. Use a dropper to add drops of deionized water, doing this ever so
carefully so it does not overfill the line (at eyelevel is best). If so, please
repeat previous steps from 1 all over again.
9. Get the solution checked by a member of staff present, then put the lid in
the volumetric flask and invert it up and down 5-10 times, to ensure all
contents are mixed. Label it NaHC03.
Titration Part 13:
1. Pour approximately 100cm3 of the standard solution (NaHCO3) of known
concentration into a clean, dry beaker.
2. Fill the burette with the standard solution of known concentration (label
this HCl)
3. Pour approximately 100cm3 of the solution with unknown concentration
into a second beaker.
4. Make 250ml of a 0.1M solution of sodium hydrogen carbonate. (Get
calculations checked)