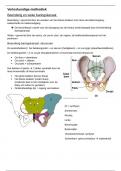
4.1 Exothermic and Endothermic Reactions:
4.1 exothermic and endothermic reactions
- When a chemical reaction takes place, chemical bonds break
and new ones are formed. 4.2 enthalpy
- Energy must be put in to break bonds and energy is given out 4.3 measuring enthalpy changes
when bonds are formed.
- The overall change may result in energy being given out or 4.4 Hess’s law
taken in. At the end if energy has been given out it is 4.5 Enthalpy changes of combustion
exothermic, if it has been taken in it is endothermic.
4.6 representing thermochemical cycles
4.2 Enthalpy: 4.7 bond enthalpies
- Enthalpy is a measure of the heat energy in a chemical
system.
- Enthalpy change ΔH – is the heat energy exchanged with the surroundings during a
reaction at a constant pressure.
- ΔH = Hproducts - Hreactants
- There are standard conditions for measuring enthalpy changes:
o Pressure of 100kPa
o Temperature of 298K
o Any solutions at a concentration of 1.0 moldm-3
- ΔH is negative for exothermic reactions and positive for endothermic reactions.
- Enthalpy level diagrams represent enthalpy changes
- The arrows only have heads at one end
o The ΔH arrow always points from reactants to products
o The Ea arrow always points upwards
- Activation energy – the minimum energy reactants must have to react.
4.3 Measuring Enthalpy Changes:
- The standard molar enthalpy of formation ∆! 𝐻∅ is the enthalpy change when one mole of substance
is formed from its constituent elements under standard conditions, all reactants and products being in
their standard states.
- The standard molar enthalpy of combustion ∆# 𝐻∅ is the enthalpy change when one mole of substance
is completely burnt in oxygen under standard conditions, all reactants and products being in their
standard states.
- To measure enthalpy change you arrange for the heat to be transferred into a particular mass of a
substance, often water. You need to know:
o Mass of the substance that is being heated up or cooled down
o Temperature change
o Specific heat capacity of the substance
- There are three main variations of enthalpy experiments.
o A substance is burnt, and the heat released is used to warm up water in a calorimeter. (Using a
spirit burner)
o Two solutions are combined together, and the head released/absorbed will change the
temperature of the solution. (Using a polystyrene cup/calorimeter)
o A solid can be added to a solution and the heat released/absorbed will change the temperature
of the solution. (Using a polystyrene cup/calorimeter)
- The temperature is monitored over a period of time, normally a few minutes before starting the
reaction. It will be stirred.
- Heat Transferred (J) = mass of substance being heated (g) x specific heat capacity (usually 4.18Jg-1K-1)
x temperature change (K/oC)