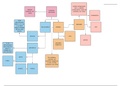
Structural isomers
Chain isomers
Chain isomers are a structural isomer and are compounds that have the same molecular
formula, but their longest hydrocarbon chain is not the same which is caused by branching.
Chain isomers happen in carbon compounds with more than four carbons. An example of this
would be pentane à2,2 dimethyl propane. Pentane consists of no side chains allowing the
compound to have strong van der walls forces, increasing the boiling point of pentane.
However, 2,2 dimethyl propane contains branching which causes the van der walls to be weaker,
decreasing the boiling point.
à
physical properties of butane
Pentane 2,2-dimethyl propane
and 2-methylpropane are
similar. The main
difference is the boiling point,
where butane has a higher
,boiling point than 2-
methylpropane.
The reason butane has a higher
boiling point is because there
are stronger intermolecular
forces, due to having a linear
carbon chain, which means
more energy is needed to
separate the
bonds between atoms.
physical properties of butane
and 2-methylpropane are
similar. The main
, difference is the boiling point,
where butane has a higher
boiling point than 2-
methylpropane.
The reason butane has a higher
boiling point is because there
are stronger intermolecular
forces, due to having a linear
carbon chain, which means
more energy is needed to
separate the
bonds between atoms.
The physical properties of pentane and 2,2-dimethyl propane have a few differences. However,
one of the main differences is that the boiling point since pentane has a higher boiling point
compared to 2,2 dimethyl propane because of the strong intermolecular forces present from
the long carbon chain which requires more energy to separate the bonds between the atoms.
Physical property Pentane 2,2 dimethyl propane
Molecular formula C 5 H 12 C 5 H 12