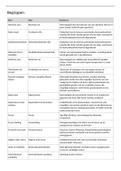
Unit 4: Laboratory Techniques and their Application
Learning aim D:Learning aim B: Explore the manufacturing techniques and
testing methods for an organic liquid (Nail varnish remover)
The method of creating ethyl ethanoate is as follows;
The reactants needed are ethanol and ethanoic acid, The catalyst needed is concentrated
sulfuric acid.The reaction is set up by adding a few drops of concentrated sulfuric acid to a
mixture of ethanol and ethanoic acid in a round-bottomed flask. The flask is then attached
to a condenser and heated gently in a water bath.The concentrated sulfuric acid acts as a
catalyst, which means it speeds up the reaction without being used up. It does this by
providing a pathway with a lower activation energy for the reaction to occur.
After the reaction is complete, the mixture is poured into a separating funnel. The ethyl
ethanoate, being less dense, forms the top layer. The lower layer, which is a mixture of
water and the excess reactants, is drained off. The ethyl ethanoate is then washed with
water to remove any remaining impurities.The ethyl ethanoate is purified by distillation.
This involves heating the ethyl ethanoate to its boiling point and collecting the vapours,
which are then condensed back into a liquid. The process of distillation helps to separate
the ethyl ethanoate from any other substances that may be present.
CH3COOH+C2H5OH↔CH3COOC2H5+H2O
The reaction that produces ethyl ethanoate is the esterification of ethanol and ethanoic
acid.The conditions that favour the production of ethyl ethanoate are high concentrations
of ethanol and ethanoic acid, low concentrations of water, and the presence of a catalyst
(usually concentrated sulfuric acid).If the concentration of one of the reactants or products
is increased, the equilibrium will shift to the side with fewer moles of gas (if the number of
moles of gas is the same on both sides, the equilibrium will not shift). If the concentration
of one of the reactants or products is decreased, the equilibrium will shift to the side with
more moles of gas (if the number of moles of gas is the same on both sides, the equilibrium
will not shift). If the pressure is increased, the equilibrium will shift to the side with fewer
moles of gas (if the number of moles of gas is the same on both sides, the equilibrium will
not shift). If the pressure is decreased, the equilibrium will shift to the side with more
moles of gas (if the number of moles of gas is the same on both sides, the equilibrium will
not shift). If the temperature is increased, the equilibrium will shift in the endothermic
direction. If the temperature is decreased, the equilibrium will shift in the exothermic
direction.To produce more ethyl ethanoate, we can increase the concentrations of ethanol
and ethanoic acid, decrease the concentration of water, and use a catalyst. Le Chatelier's
principle is applied to the reaction. Le Chatelier's principle states that if a system at
equilibrium is subjected to a change, the equilibrium will shift in the direction that
minimises the change.
1
, Factors affecting yield and purity.
The factors that can affect the yield of the reaction include the concentration of the
reactants, the temperature, the pressure, the presence of a catalyst, and the reaction time
.- Concentration of the reactants: Increasing the concentration of the reactants will
increase the yield of the reaction. - Temperature: Increasing the temperature will increase
the rate of the reaction, but it may also cause the reaction to become less selective, leading
to the formation of other products and reducing the yield of the desired product. -
Pressure: Increasing the pressure will increase the yield of the reaction, but this is not a
significant factor in this particular reaction as it is not a gas-phase reaction. - Catalyst: The
catalyst increases the rate of the reaction, but it does not affect the yield. - Reaction time:
Increasing the reaction time will increase the yield of the reaction, but it will also increase
the time required for the reaction to complete.
The factors that can affect the purity of the ethyl ethanoate include the presence of
impurities in the reactants, the reaction conditions, and the separation and purification
methods used.- Impurities in the reactants: If the reactants are not pure, they can
introduce impurities into the product.
- Reaction conditions: The reaction conditions can affect the selectivity of the reaction,
leading to the formation of other products and reducing the purity of the desired product.
- Separation and purification methods: The methods used to separate and purify the
product can affect its purity. For example, if the product is not properly distilled,
impurities may remain in the final product. The significance of these factors will depend
on the specific conditions of the reaction. For example, if the reactants are not pure, this
could significantly affect the purity of the product. However, if the reactants are pure and
the reaction is carefully controlled, the other factors may have less of an impact.
In our reaction, some impurities could be due to the age, grade or condition of the reactant
chemicals we used.
To calculate the percentage yield i did the following calculations:
Mass = Density x Volume = 52.5 —> Mol = Mass / Mr = 0.875
% yield = actual yield / theoretical yield
0..875 x 100 = 28.25169837
Percentage yield = 28.25169837
2
Learning aim D:Learning aim B: Explore the manufacturing techniques and
testing methods for an organic liquid (Nail varnish remover)
The method of creating ethyl ethanoate is as follows;
The reactants needed are ethanol and ethanoic acid, The catalyst needed is concentrated
sulfuric acid.The reaction is set up by adding a few drops of concentrated sulfuric acid to a
mixture of ethanol and ethanoic acid in a round-bottomed flask. The flask is then attached
to a condenser and heated gently in a water bath.The concentrated sulfuric acid acts as a
catalyst, which means it speeds up the reaction without being used up. It does this by
providing a pathway with a lower activation energy for the reaction to occur.
After the reaction is complete, the mixture is poured into a separating funnel. The ethyl
ethanoate, being less dense, forms the top layer. The lower layer, which is a mixture of
water and the excess reactants, is drained off. The ethyl ethanoate is then washed with
water to remove any remaining impurities.The ethyl ethanoate is purified by distillation.
This involves heating the ethyl ethanoate to its boiling point and collecting the vapours,
which are then condensed back into a liquid. The process of distillation helps to separate
the ethyl ethanoate from any other substances that may be present.
CH3COOH+C2H5OH↔CH3COOC2H5+H2O
The reaction that produces ethyl ethanoate is the esterification of ethanol and ethanoic
acid.The conditions that favour the production of ethyl ethanoate are high concentrations
of ethanol and ethanoic acid, low concentrations of water, and the presence of a catalyst
(usually concentrated sulfuric acid).If the concentration of one of the reactants or products
is increased, the equilibrium will shift to the side with fewer moles of gas (if the number of
moles of gas is the same on both sides, the equilibrium will not shift). If the concentration
of one of the reactants or products is decreased, the equilibrium will shift to the side with
more moles of gas (if the number of moles of gas is the same on both sides, the equilibrium
will not shift). If the pressure is increased, the equilibrium will shift to the side with fewer
moles of gas (if the number of moles of gas is the same on both sides, the equilibrium will
not shift). If the pressure is decreased, the equilibrium will shift to the side with more
moles of gas (if the number of moles of gas is the same on both sides, the equilibrium will
not shift). If the temperature is increased, the equilibrium will shift in the endothermic
direction. If the temperature is decreased, the equilibrium will shift in the exothermic
direction.To produce more ethyl ethanoate, we can increase the concentrations of ethanol
and ethanoic acid, decrease the concentration of water, and use a catalyst. Le Chatelier's
principle is applied to the reaction. Le Chatelier's principle states that if a system at
equilibrium is subjected to a change, the equilibrium will shift in the direction that
minimises the change.
1
, Factors affecting yield and purity.
The factors that can affect the yield of the reaction include the concentration of the
reactants, the temperature, the pressure, the presence of a catalyst, and the reaction time
.- Concentration of the reactants: Increasing the concentration of the reactants will
increase the yield of the reaction. - Temperature: Increasing the temperature will increase
the rate of the reaction, but it may also cause the reaction to become less selective, leading
to the formation of other products and reducing the yield of the desired product. -
Pressure: Increasing the pressure will increase the yield of the reaction, but this is not a
significant factor in this particular reaction as it is not a gas-phase reaction. - Catalyst: The
catalyst increases the rate of the reaction, but it does not affect the yield. - Reaction time:
Increasing the reaction time will increase the yield of the reaction, but it will also increase
the time required for the reaction to complete.
The factors that can affect the purity of the ethyl ethanoate include the presence of
impurities in the reactants, the reaction conditions, and the separation and purification
methods used.- Impurities in the reactants: If the reactants are not pure, they can
introduce impurities into the product.
- Reaction conditions: The reaction conditions can affect the selectivity of the reaction,
leading to the formation of other products and reducing the purity of the desired product.
- Separation and purification methods: The methods used to separate and purify the
product can affect its purity. For example, if the product is not properly distilled,
impurities may remain in the final product. The significance of these factors will depend
on the specific conditions of the reaction. For example, if the reactants are not pure, this
could significantly affect the purity of the product. However, if the reactants are pure and
the reaction is carefully controlled, the other factors may have less of an impact.
In our reaction, some impurities could be due to the age, grade or condition of the reactant
chemicals we used.
To calculate the percentage yield i did the following calculations:
Mass = Density x Volume = 52.5 —> Mol = Mass / Mr = 0.875
% yield = actual yield / theoretical yield
0..875 x 100 = 28.25169837
Percentage yield = 28.25169837
2