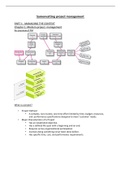
subject title check
22 reaction kinetics date
rate of reaction
the units of k vary change in amount/concentration of a reactant/product per unit time
N
according to the form -rate of reaction k(A) (B) where k rate constant
M
-
of the rate equatoin (A) and (B) conc. of reactants
-
m and n orders of the reaction
-
order of reaction
the power to which the concentration of the reactant is raised in
the rate equation (concentration is ignored if order is 0)
-overall order of reaction
the sum of powers of the reactants in a rate equation
concentration-time graph initial rates
&
to find the rate of
reaction, draw a
tangent and
calculate the slope
half-life (t1/2)
the time taken for the amount/concentration of the limiting
reactant in a reaction to decrease to half its initial value
half-life of a first-order reaction is independent of the
concentration of reactants
calculating the rate constant
-using initial rates and the rate equation
substitute the values of initial rate and concentrations to the
rate equation
Copyright 2021. 해움. All rights reserved.
22 reaction kinetics date
rate of reaction
the units of k vary change in amount/concentration of a reactant/product per unit time
N
according to the form -rate of reaction k(A) (B) where k rate constant
M
-
of the rate equatoin (A) and (B) conc. of reactants
-
m and n orders of the reaction
-
order of reaction
the power to which the concentration of the reactant is raised in
the rate equation (concentration is ignored if order is 0)
-overall order of reaction
the sum of powers of the reactants in a rate equation
concentration-time graph initial rates
&
to find the rate of
reaction, draw a
tangent and
calculate the slope
half-life (t1/2)
the time taken for the amount/concentration of the limiting
reactant in a reaction to decrease to half its initial value
half-life of a first-order reaction is independent of the
concentration of reactants
calculating the rate constant
-using initial rates and the rate equation
substitute the values of initial rate and concentrations to the
rate equation
Copyright 2021. 해움. All rights reserved.