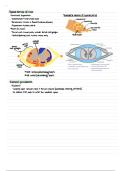
Unit 5 Flame Test NaOH NaOH + Time Excess NaOH Na2CO3 Na2SO4 Conc HCl NH3 Excess NH3 KI KI + Time H2SO4
Group 2 Mg 2+ White ppt White ppt Colourless sol
Ca 2+ Brick Red White ppt White ppt White ppt
Sr 2+ Crimson Some white ppt White ppt White ppt
Ba 2+ Apple Green Thin white ppt White ppt White ppt
Group 1 Li + Red All soluble
Na + Yellow/orange Colourless
K+ Lilac solution
Complexes [Cu(H2O)6]2+ [Cu(H2O)4(OH)2] [CuCl4]2- [Cu(H2O)4(OH)2] [Cu(NH3)4(H2O)2]2+ CuI + I2 CuI + I2
Blue/green Pale blue ppt Green sol Pale blue ppt Royal blue sol Brown sol & white ppt
[Co(H2O)6]2+ [Co(H2O)4(OH)2] [Co(H2O)4(OH)2] [CoCl4]2-
Pale pink sol Blue ppt PInk ppt Dark blue sol
AlCl3 Al(OH)3 [Al(OH)4]-
Colourless sol White ppt Colourless sol
Pb(NO3)2 Pb(OH)2 [Pb(OH)4]2- PbCl2 PbI2 PbSO4
Colourless sol White ppt Colourless sol Heavy White ppt Bright yellow ppt White ppt
TM [Cr(H2O)6]3+ [Cr(H2O)3(OH)3] [Cr(OH)6]3-
Blue/purple sol Pale green ppt Dark green
[Fe(H2O)6]2+ [Fe(H2O)4(OH)2]
Pale green sol Dark green ppt
[Fe(H2O)6]3+ [Fe(H2O)3(OH)3]
Yellow sol Red/brown ppt
Group 2 Mg 2+ White ppt White ppt Colourless sol
Ca 2+ Brick Red White ppt White ppt White ppt
Sr 2+ Crimson Some white ppt White ppt White ppt
Ba 2+ Apple Green Thin white ppt White ppt White ppt
Group 1 Li + Red All soluble
Na + Yellow/orange Colourless
K+ Lilac solution
Complexes [Cu(H2O)6]2+ [Cu(H2O)4(OH)2] [CuCl4]2- [Cu(H2O)4(OH)2] [Cu(NH3)4(H2O)2]2+ CuI + I2 CuI + I2
Blue/green Pale blue ppt Green sol Pale blue ppt Royal blue sol Brown sol & white ppt
[Co(H2O)6]2+ [Co(H2O)4(OH)2] [Co(H2O)4(OH)2] [CoCl4]2-
Pale pink sol Blue ppt PInk ppt Dark blue sol
AlCl3 Al(OH)3 [Al(OH)4]-
Colourless sol White ppt Colourless sol
Pb(NO3)2 Pb(OH)2 [Pb(OH)4]2- PbCl2 PbI2 PbSO4
Colourless sol White ppt Colourless sol Heavy White ppt Bright yellow ppt White ppt
TM [Cr(H2O)6]3+ [Cr(H2O)3(OH)3] [Cr(OH)6]3-
Blue/purple sol Pale green ppt Dark green
[Fe(H2O)6]2+ [Fe(H2O)4(OH)2]
Pale green sol Dark green ppt
[Fe(H2O)6]3+ [Fe(H2O)3(OH)3]
Yellow sol Red/brown ppt