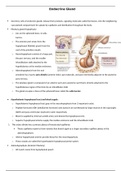
Exothermic and endothermic reactions
When a chemical reaction occurs, energy is transferred to or from the surroundings and there is
usually a temperature change, when a there is a fire burning, it transfers heat energy to the
surroundings. The objects that are near the fire become warmer the temperature change can be
measured with a thermometer.
Exothermic reactions are reactions that transfer energy to the surroundings. The energy
transferred is usually heat energy, which causes the reaction mixture and the surroundings to get
warmer. The increase in temperature can be measured with a thermometer. Eventually the
temperature of the products decreases as they lose heat energy.
Examples of exothermic reactions are:
• Burning (combustion)
Charcoal burning
A candle burning
Firework exploding
• neutralisation reactions between acids and alkalis,
• the reaction between water and calcium oxide
(Arrington)
In exothermic reactions the energy of the reactants is greater than the energy of the products. The
activation energy is the minimum energy required to break the bonds and start the reaction off.
When a chemical reaction occurs, energy is transferred to or from the surroundings and there is
usually a temperature change, when a there is a fire burning, it transfers heat energy to the
surroundings. The objects that are near the fire become warmer the temperature change can be
measured with a thermometer.
Exothermic reactions are reactions that transfer energy to the surroundings. The energy
transferred is usually heat energy, which causes the reaction mixture and the surroundings to get
warmer. The increase in temperature can be measured with a thermometer. Eventually the
temperature of the products decreases as they lose heat energy.
Examples of exothermic reactions are:
• Burning (combustion)
Charcoal burning
A candle burning
Firework exploding
• neutralisation reactions between acids and alkalis,
• the reaction between water and calcium oxide
(Arrington)
In exothermic reactions the energy of the reactants is greater than the energy of the products. The
activation energy is the minimum energy required to break the bonds and start the reaction off.