- London forces ∴ low BPT (80°C) Aromatics = containing benzene
- Insoluble in H2O Derivatives of benzene ----------->
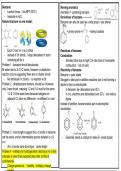
Kekule structure vs new model; Benzene can also be used as a side group = use phenyl
= phenyl amine =phenyl acetate
- Each C has 1e- in a p orbital Reactions of benzene:
- Instead of 3π bonds, 1 large delocalised π bond Combustion
containing all 6e-’s - Smokey flame due to high C:H ratio (lots of incomplete
Problem 1 - benzene should decolourise combustion ∴ lots of soot)
Br water due to 3x C=C bonds, however a substitution Reactivity of benzene:
reaction occurs suggesting there are no double bonds. Benzene = quite stable
- No individual c=c bonds ∴ no reaction w/ Br Struggle to take part in addition reactions due to not forming a
Problem 2 - dihalobenzene isomers, should be 4 however dipole to form an electrophile
only 3 were found, meaning 1-2 and 1-6 must be the same. - In benzene, 6e- delocalised over 6C’s
- 1-2 & 1-6 the same bond because halogens on - In c=c, electrons are delocalised over 2C’s ∴ can induce
adjacent C’s have no difference - no different c-c and dipole
c=c bonds. Instead of addition, benzene takes part in electrophilic
substitution:
Problem 3 - bond lengths suggest the c-c bonds in benzene
are the same, and an intermediate species between c-c & - Generally needs a catalyst to make E+ (need dipole)
c=c
- All c-c bonds same bond type ∴ same length
Problem 4 - enthalpy for hydrogenation (reacting w H 2 to fully
saturate) is lower than expected (less than 3x that of
cyclohexane)
- Charge spread out ∴ ↑stability ∴enthalpy change
, Reactions of benzene (continued) Friedel-crafts reactions Phenols:
Nitration: = attaching extra C atoms to a benzene ring = an OH on a benzene
- Reagents = conc H2SO4 & conc HNO3 Alkylation: ‘aromatic alcohols’
- Conditions = 50°C
Phenol = pink solid at
Acylation: room temp
- Conditions = under reflux Benzene rings in
phenol:
The non-bonding e-’s on
the O in OH are drawn
into the ring
→
↑ e- density in the ring
∴↑ susceptible to
electrophilic attack
Reduction of nitrobenzene: Further substitution of arenes (containing
Properties of phenols:
- Reagents = tin & conc HCl (reducing agents) benzene)
- high MPT (H bonds)
- Conditions = heat under reflux Electron withdrawing groups - soluble-ish (H bonds)
- Substitution of nitrobenzene - weak acid:
= harder than w/ benzene
- Takes e-’s out of the ring
- ∴ harder to react
- Produces are 1,3-disubstituted product
Halogenation: Electron donating groups (eqm lies on LHS ∴weak)
- Reagents = AlCl3 or FeBr3 - Substitution of methylbenzene - ↑ reactive than benzene
- Conditions = room temp - Reacts w/ HNO3
= easier than w/ benzene
without catalyst
- Pushes e- density into the ring - Reacts w/ Br without
- ∴ easier to react a catalyst
- Produces a mix of 1,2 and 1,4
disubstituted products