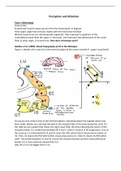
Scientist Experiment Sketch of Model Description of Model
Democritus argely observations
L ifferent sizes,
D
Broke shells into smaller pieces shapes, and colours
Dalton as laws
G illiard ball model
B
No empirical test of atomic Invisible solid spheres
theory
Thompson athode Ray Experiment
C lum Pudding Model
P
Beam attracted / repelled to a Electrons floating in
magnet cloud of positive
charge
rnest
E old Foil Experiment
G lanetary Model
P
Rutherford Alpha particles shot at a gold foil Positive charge is
99% passed straight through concentrated in the
Small % deflected due to centre
repulsion between charges
etermined that atoms are
D
largely empty space w/
negatively charged electrons that
surround a dense, positively
charged nucleus
Bohr ydrogen emission spectrum
H lectron shell / energy
E
Only certain wavelengths are level
observed Electrons are fixed
distances from the
nucleus
Subatomic particles: protons (p+), electrons (e-), neutrons (n⁰)
, tomic number = # of protons
A
Mass number = # of protons + # of
neutrons
egative Ion (anion): when atom
N
gains electrons
Positive ion (cation): when atom loses
electrons
Isotopes: atoms of the same element that have the same atomic #, but different mass # (same #
of protons, different numbers of neutrons) i.e. isotopew/ mass # 12 -> carbon-12
ame chemical properties, slightly different physical properties (mass # ↑, melting point ↑,
S
boiling point ↑, density ↑)
Relative atomic mass (𝐴𝑟) - weighted average of an element’s naturally occurring isotopes
Percent abundance - percent of an isotope in a naturally occurring sample of an element
Relative atomic mass is likely closest to the isotope with the most percent abundance.
M
● ass spectrometer detects the natural abundance of isotopes in a sample
● Degree of deflection = mass to charge (m/z) ratio
, ○ Low m & high z is deflected the most
elative intensity - the size of a peak in a mass spectrum relative to the most abundant ion
R
which is shown as the tallest peak in the spectrum. The most abundant ion has intensity 100
(base peak)
● In a mass spectra (graph of mass spectrometer data), if there are more peaks than
expected, it can be because some molecules have 2 of the same isotope / a
combination of each
, 1.3 ELECTRON CONFIGURATIONS
avelength (𝛌) is inversely proportional
W
to frequency (𝑓)
Speed of light in vacuum (c) = 3.00 x 10⁸
𝑐 = λ𝑓 (all regions of EM spectrum are
the same speed)
mission line spectrum - produced when
E
energy is applied to an element and
viewed through a spectroscope.
Name Produced by Image Description Electron Movement
ontinuous Black body
C hows all
S N/A
Spectrum wavelengths of
visible light
mission
E Hot gas hows specific 1
S . Electrons are excited to
Line wavelengths of higher energy level
Spectrum visible light
2. Return to a lower
Unique to each energy level
element
3. Release a photon of
Lines converge specific energy
at higher
bsorption
A Cold gas energy levels 1. Electrons are excited to
Line higher energy level
Spectrum
. Absorb photons of a
2
specific energy
lectrons are in orbits around the nucleus
E
Orbits are associated with discrete energy
levels
Energy levels converge at higher energy
round state: lowest energy level of an atom
G
when electrons are at their lowest energy levels
xcited state (unstable): highest energy level of
E
an atom when electrons gain energy and move
to higher energy levels