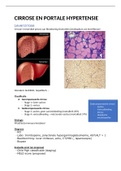
Inorganic Ions
Metal Aqua Ions
When in aqueous solution withou the presence of other ions, metal ions exist as metal aqua ions
Metal Aqua Ion – a central metal ion surrounded by 6 water ligands
When salts are crystallised from solution, metal aqua ions are often present in the crystals
o Blue crystals of hydrated copper(II) sulfate due to [Cu(H 2O)6]2+
Aqueous Ion Complexes
o [Cu(H2O)6]2+ – Blue
o [Fe(H2O)6]2+ – Green
o [Al(H2O)6]3+ – Colourless, not a transition metal
o [Fe(H2O)6]3+ – Pale violet crystals, yellow-orange in solution due to presence of small
amounts of [Fe(H2O)5(OH)]2+
Reactions of Metal Aqua Ions
Hydrolysis – loss of H+ from H2O ligands, O-H bond in H2O ligand breaks
Substitution – replacement of H2O by other ligands, metal-ligand bond breaks
Redox metal changes oxidation state – gain or loss of electrons
Hydrolysis
In solution, metal aqua ions lose H+ from one or more H2O ligands in a hydrolysis reaction
Hydrolysis of M2+ aqua ions
o [M(H2O)6]2+ ⇋ [M(H2O)5(OH)]+ + H+
o pH of ~6
o Equilibrium lies to the left, not many H+ released
Hydrolysis of M3+ aqua ions
o [M(H2O)6]3+ ⇋ [M(H2O)5(OH)]2+ + H+
o pH of 3
o M3+ ion is smaller and more highly charged than M 2+ so the O-H bond breaks more easily
Further Hydrolysis to Form Precipitates
Metal aqua 3+ ions
o [M(H2O)6]3+ ⇋ [M(H2O)5(OH)]2+ + H+
o [M(H2O)5(OH)]2+ ⇋ [M(H2O)4(OH)2]+ + H+
o [M(H2O)4(OH)2]+ ⇋ [M(H2O)3(OH)3] + H+ (precipitate)
Metal aqua 2+ ions
o [M(H2O)6]2+ ⇋ [M(H2O)5(OH)]+ + H+
o [M(H2O)5(OH)]+ ⇋ [M(H2O)4(OH)2] + H+ (precipitate)
Reaction with Bases
If a base is added to the metal aqua ion, hydrolysis may take place
o The base removes the H+, pushing the equilibria right
o Common bases are OH–, NH3, CO32–
Metal Aqua Ions
When in aqueous solution withou the presence of other ions, metal ions exist as metal aqua ions
Metal Aqua Ion – a central metal ion surrounded by 6 water ligands
When salts are crystallised from solution, metal aqua ions are often present in the crystals
o Blue crystals of hydrated copper(II) sulfate due to [Cu(H 2O)6]2+
Aqueous Ion Complexes
o [Cu(H2O)6]2+ – Blue
o [Fe(H2O)6]2+ – Green
o [Al(H2O)6]3+ – Colourless, not a transition metal
o [Fe(H2O)6]3+ – Pale violet crystals, yellow-orange in solution due to presence of small
amounts of [Fe(H2O)5(OH)]2+
Reactions of Metal Aqua Ions
Hydrolysis – loss of H+ from H2O ligands, O-H bond in H2O ligand breaks
Substitution – replacement of H2O by other ligands, metal-ligand bond breaks
Redox metal changes oxidation state – gain or loss of electrons
Hydrolysis
In solution, metal aqua ions lose H+ from one or more H2O ligands in a hydrolysis reaction
Hydrolysis of M2+ aqua ions
o [M(H2O)6]2+ ⇋ [M(H2O)5(OH)]+ + H+
o pH of ~6
o Equilibrium lies to the left, not many H+ released
Hydrolysis of M3+ aqua ions
o [M(H2O)6]3+ ⇋ [M(H2O)5(OH)]2+ + H+
o pH of 3
o M3+ ion is smaller and more highly charged than M 2+ so the O-H bond breaks more easily
Further Hydrolysis to Form Precipitates
Metal aqua 3+ ions
o [M(H2O)6]3+ ⇋ [M(H2O)5(OH)]2+ + H+
o [M(H2O)5(OH)]2+ ⇋ [M(H2O)4(OH)2]+ + H+
o [M(H2O)4(OH)2]+ ⇋ [M(H2O)3(OH)3] + H+ (precipitate)
Metal aqua 2+ ions
o [M(H2O)6]2+ ⇋ [M(H2O)5(OH)]+ + H+
o [M(H2O)5(OH)]+ ⇋ [M(H2O)4(OH)2] + H+ (precipitate)
Reaction with Bases
If a base is added to the metal aqua ion, hydrolysis may take place
o The base removes the H+, pushing the equilibria right
o Common bases are OH–, NH3, CO32–