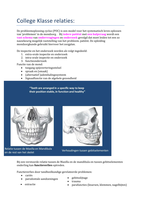
Ozone layer
-UV light breaks oxygen molecules into oxygen radicals
- Chlorofluorocarbons (CFCs) are common compounds found in aerosol
propellants. When entering the stratosphere, they break down the ozone
layer.
Initiation:
Conditions: UV light
Type of fission: homolytic (when both bonding atoms receive one electron each from
the bonded atoms).
C-Cl breaks first as it has the lowest bond enthalpy, so less energy is needed to
overcome the bond.
Propagation:
Termination:
Overall equation:
-UV light breaks oxygen molecules into oxygen radicals
- Chlorofluorocarbons (CFCs) are common compounds found in aerosol
propellants. When entering the stratosphere, they break down the ozone
layer.
Initiation:
Conditions: UV light
Type of fission: homolytic (when both bonding atoms receive one electron each from
the bonded atoms).
C-Cl breaks first as it has the lowest bond enthalpy, so less energy is needed to
overcome the bond.
Propagation:
Termination:
Overall equation: