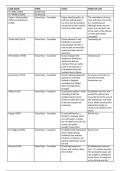
25.1 Naming Organic Compounds:
25.1 Naming organic compounds
Priority Family Suffix when Prefix when not 25.2 Optical isomerism
priority priority
25.3 synthesis of optically active compounds
1 Carboxylic acid -oic acid
2 Esters -yl /-oate
3 Amides -amide
4 Aldehydes -al Oxo-
5 Ketones -one Oxo-
6 Alcohol -ol Hydroxy-
7 Amine -amine Amino-
8 Alkenes/Alkynes -ene/-yne en-/yn-
9 Haloalkanes Fluoro-, chloro-
10 Alkanes -ane Methyl-, ethyl-
25.2 Optical Isomerism:
- Stereoisomerism is where compounds have the same structural formula, but they are arranged
differently in space. There are 2 types
o E-Z isomerism
o Optical isomerism
- Optical isomerism occurs when 4 different groups are attached to a carbon (called the chiral centre),
the isomers are a mirror image and non-superimposable.
- Optical isomers have identical chemical and physical properties, except:
o The way they rotate a plane of light
o Their reaction with other chiral molecules
- A pair of optical isomers are referred to as enantiomers.
- Optical rotation can be measured using a polarimeter, one will rotate the plane of polarisation clockwise
(this is the + isomer) and one will rotate it anticlockwise (this is the – isomer)
- All alpha amino acids are chiral molecules, because the central carbon is attached to 4 different groups
(COOH, NH2, R, H).
- A 50/50 mixture of the two enantiomers is called a racemic mixture or a racemate.
25.3 Synthesis of Optically Active Compounds:
- Some drugs are optically active molecules, many drugs work by a molecule fitting into a cell receptor,
because receptors have a 3D structure only one isomer will fit.
- Sometimes one isomer is an effective drug and the other is inactive. There are 3 solutions:
o Separate the two isomers – this can be difficult and expensive as they have such similar
properties
o Sell the mixture – wasteful as half is inactive
o Design an alternative synthesis that only makes the required isomer.
- Sometimes one isomer is an effective drug and the other is toxic or has bad side effects.
- Ibuprofen is sold as a racemate, the non-active isomer is converted to the active one in the body by
enzymes.
- Thalidomide is an optical isomer, one is a safe sedative, the other causes birth defects