CHEMISTRY
Teacher:
Location:
, Table of contents
.................................................................................................. 1
Chemistry in society ..................................................... 4
Controlling the rates ................................................................... 4
Factors that affect the rate of reaction ................................................................................................................................... 4
Catalysts .......................................................................................................................................................................................................... 5
Calculating the average rate of reaction.................................................................................................................................. 5
Calculating the relative rate ............................................................................................................................................................... 5
Collision theory.............................................................................................................................................................................................. 5
Collision geometry ...................................................................................................................................................................................... 5
Reaction profiles .......................................................................................................................................................................................... 6
Exothermic Reactions .............................................................................................................................................................................. 6
Endothermic reactions............................................................................................................................................................................. 6
Potential energy diagrams.................................................................................................................................................................... 6
Reversible Reactions ................................................................................................................................................................................ 7
Activation energy ........................................................................................................................................................................................ 7
The activated complex ............................................................................................................................................................................ 7
Temperature and kinetic energy ...................................................................................................................................................... 7
Catalysts and Potential energy diagrams................................................................................................................................. 8
Catalysts and energy distribution ................................................................................................................................................... 8
Chemical Energy ............................................................................. 8
Bonds ................................................................................................................................................................................................................... 8
Measuring enthalpy changes ............................................................................................................................................................... 9
Enthalpy of combustion of alcohols ............................................................................................................................................... 9
Bond enthalpies ............................................................................................................................................................................................. 9
Equilibrium ........................................................................................... 9
Le chatliers principle ...............................................................................................................................................................................10
Changing the concentration ...............................................................................................................................................................10
Changing the temperature ..................................................................................................................................................................10
,Changing the pressure ........................................................................................................................................................................... 11
Catalysts effect on equilibrium ......................................................................................................................................................... 11
Getting the most from reactants ....................................... 12
Avogadro’s constant .............................................................................................................................................................................. 12
Mole ratios ..................................................................................................................................................................................................... 12
Molar gas volume calculations ....................................................................................................................................................... 12
Calculating the excess reactant ....................................................................................................................................................13
Calculating the limiting reactant.....................................................................................................................................................13
Percentage yield .........................................................................................................................................................................................13
Atom economy ........................................................................................................................................................................................... 14
Chemical industry ..................................................................................................................................................................................... 14
Types of reactions ...................................................................... 16
Combination reaction ............................................................................................................................................................................. 17
Decomposition reaction......................................................................................................................................................................... 17
Electrolytic decomposition (electrolysis)................................................................................................................................... 17
Photo decomposition (photolysis) ................................................................................................................................................... 17
Displacement reaction (substitution/replacement)............................................................................................................ 17
Neutralisation............................................................................................................................................................................................... 17
Oxidation.......................................................................................................................................................................................................... 17
Reduction ......................................................................................................................................................................................................... 17
Redox ................................................................................................................................................................................................................. 17
Precipitation ................................................................................................................................................................................................... 18
Hydrolysis ........................................................................................................................................................................................................ 18
hydration.......................................................................................................................................................................................................... 18
, Unit 3
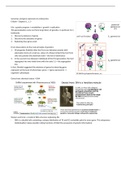
Factors that affect the rate of
reaction
- Temperature of the experiment
The higher the temperature, the more
particles will have the activation energy
- Particle size of the reactant
required for reaction to occur.
The smaller the particles, the faster the
Higher temperature also causes
reaction because smaller particles
molecules to move faster which leads
provide more surface area.
to more collisions.
(The rate of reaction doubles for every
10°c increase in temperature.)
- Concentration of the reactant
The higher the concentration, the faster
the reaction because there will be more
particles crowded into a smaller
volume, so particles are more likely to - The use of a catalyst
collide with each other.
Catalyst lower the activation energy
required for a reaction to occur
meaning less energy is needed by
particles to cause a reaction.
4