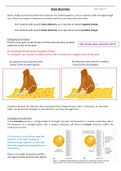
Static charge can build up when two materials are rubbed together, such as a plastic comb moving through
hair. When this happens electrons are transferred from one material to the other:
- One material ends up with more electrons, so it now has an overall negative charge.
- One material ends up with fewer electrons, so it now has an overall positive charge.
Charging via Friction
Protons never get transferred due to friction because they are deep
Like charges repel, opposites attract
within the centre of the atom.
An insulating material can be charged by friction.
For example, if an insulator is rubbed with a cloth, it can become charged in one of two ways:
A. Electrons move from the cloth B. Electrons move from the insulator
(loses) to the insulator (gains). (loses) to the cloth (gains).
Insulators prevent the electrons from moving and the charge remains static. Conductors, on the other
hand, cannot become charged, as the electrons can move through them.
Charging via Induction
In the induction process, a charged object is brought near but not touched to a neutral conducting object.
The presence of a charged object near a neutral conductor will force (or induce) electrons within the
conductor to move.
The electrons in the balloon repel the
electrons in the wall, causing an
induced charge separation in the wall,
which the negatively charged balloon is
attracted to.
There is a transfer of electrons within
one object (which polarises that object)