-
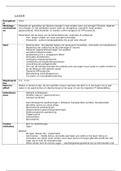
Chemical_ Equilibriar -
Open System :
System in which both
energy
and matter can be
exchanged between the system and
it's
surroundings
Cannot reach equilibrium
Closed System System in which mass is conserved but can enter leave
energy or
:
Can reach equilibrium
Reversible -
Reaction-
Products
·
can be converted back into reactants
for forward
Enthalpy change reaction
*
is
ex o
3Ha + Na 2NH3 Ho
- -
endo
-Chemical Equilibrium - -
Forward
·
and reverse reactions are at the same rate
Concentration of
-
products and reactants is equal
&
-------- Na &
---
==
&
N --- Ha
o
-------
"
- - - -
NHy
- - -
-
1- -
-
!
-
-
-
-
- -
time(s) time(s)
, _
Le _
ChateliersPrinciple-
When an external stress is applied to a
system in dynamic chemical equilibrium ,
the
equilibrium point will
change in such
ray
a as to counteract the stress
Describing a shift in chemical equilibrium :
1 the
.
Identify external stress
I
------------------
Pressure
I Catalysts increases forward and reverse
I
I
I
-
Temperature !
reactions :No infact on equilibrium
I
L
Concentration
2 State the
.
systems response
System will respond to counteract stress
.
3 State which reaction is favored and equilibrium shift
Pressure-
· Favours forward reaction
Less moles produced
·
If
----------
pressure increases -
*
exo
-Volume is decreased 3Ha + Na 2NHa Ho
endo
*
System favors reaction with fewer
-
moles
-
This
-
decreases Pressure
-
"Favours
decreased--------
reverse reaction
·
If pressure is More moles produced
Volume of container increases
System favors that
-
reaction produces more molecules
This the pressure
-
increases
Chemical_ Equilibriar -
Open System :
System in which both
energy
and matter can be
exchanged between the system and
it's
surroundings
Cannot reach equilibrium
Closed System System in which mass is conserved but can enter leave
energy or
:
Can reach equilibrium
Reversible -
Reaction-
Products
·
can be converted back into reactants
for forward
Enthalpy change reaction
*
is
ex o
3Ha + Na 2NH3 Ho
- -
endo
-Chemical Equilibrium - -
Forward
·
and reverse reactions are at the same rate
Concentration of
-
products and reactants is equal
&
-------- Na &
---
==
&
N --- Ha
o
-------
"
- - - -
NHy
- - -
-
1- -
-
!
-
-
-
-
- -
time(s) time(s)
, _
Le _
ChateliersPrinciple-
When an external stress is applied to a
system in dynamic chemical equilibrium ,
the
equilibrium point will
change in such
ray
a as to counteract the stress
Describing a shift in chemical equilibrium :
1 the
.
Identify external stress
I
------------------
Pressure
I Catalysts increases forward and reverse
I
I
I
-
Temperature !
reactions :No infact on equilibrium
I
L
Concentration
2 State the
.
systems response
System will respond to counteract stress
.
3 State which reaction is favored and equilibrium shift
Pressure-
· Favours forward reaction
Less moles produced
·
If
----------
pressure increases -
*
exo
-Volume is decreased 3Ha + Na 2NHa Ho
endo
*
System favors reaction with fewer
-
moles
-
This
-
decreases Pressure
-
"Favours
decreased--------
reverse reaction
·
If pressure is More moles produced
Volume of container increases
System favors that
-
reaction produces more molecules
This the pressure
-
increases