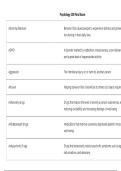
• Thermodynamics is the study of the relations
between heat, work, temperature, and energy.
• The laws of thermodynamics describe how the
energy in a system changes and whether the
system can perform useful work on its
surroundings.
• In broad terms, thermodynamics deals with the
transfer of heat energy from one
place/body/system to another.
, Difference between heat and
temperature
• Heat measures the total energy, both kinetic
and potential of the particles that make up a
body while Temperature measures the
average kinetic energy of the particles of a
body.
• SI unit of heat is a joule(J) while temperature
is kelvin (K)
, Heat transfer
• According to thermodynamic systems, heat
transfer is defined as“The movement of heat across
the border of the system due to a difference in
temperature between the system and its
surroundings.”
• Conduction is defined as the process of transmission
of energy from one particle of the medium to another
with the particles being in direct contact with each
other. Conduction is the method of transfer of heat
within a body or from one body to the other due to the
transfer of heat by molecules vibrating at their mean
positions. The bodies through which the heat transfer
must be in contact with each other. There is no actual
movement of matter while transferring heat from one
location to the other