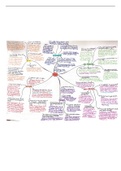
Summary Proteins, Enzymes, and Nucleic Acids
Amino Acids - The building blocks of proteins. Amino Acids - Contain a carboxylic acid group and and an amino acid group on the alpha carbon. Amino Acids - are ionized in solution. Amino Acids - Each contain a different side group (R). Nonpolar - Hydrocarbon side chains (hydrophobic) Polar (neutral) - Polar or ionic side chains (hydrophilic) Polar (acidic) - Acidic side chains (hydrophilic) Polar (basic) - -NH2 side chains (hydrophilic) H, an alkyl group, or aromatic - A nonpolar AA has an R group that is Alcohol, thiol, or amide - A polar (neutral) AA has an R group that is Carboxyl R group (COO-) - An AA is acidic with Amino R group (NH3+) - An AA is basic with Amino Acids - Chiral except glycine, which has 2 H atoms attached to the alpha C atom. Amino Acids - Have Fischer projections that are stereoisomers. Amino Acids - That are L isomers are used in proteins. Zwitterion - Has an equal number of -NH3+and COO- groups. Zwitterion - Forms when the H from -COOH in an AA transfers to the -NH2. Isoelectric points - The pH at which zwitterions have an overall zero charge. Isoelectric points - Of nonpolar and polar (neutral) AAs exist at pH values from 5.1 to 6.3. solutions that are more acidic than the pI - The COO- in the zwitterion accepts a proton. solutions that are more acidic than the pI - The AA has a positive charge. solutions that are more basic than the pI - The NH3+ in the zwitterion loses a proton. solutions that are more basic than the pI - The AA has a negative charge. 1+ charge - Glycine with a pI of 6.0 in solutions that have a pH above 6.0. 1- charge - Glycine with a pI of 6.0 in solutions that have a pH below 6.0. Polar (acidic) AAs - Zwitterions exist at pH values from 2.8 to 3.2. Polar (basic) AAs - Zwitterions exist at pH values from 7.6 to 10.8. Aspartic Acid - Has a pI of 2.8. Aspartic Acid - Forms a zwitterion at pH 2.8. Aspartic Acid - Forms negative ions with charges 1- and 2- at pH values greater than pH 2.8. Peptide bond - An amide bond Peptide bond - Forms between the carboxyl group of one AA and the amino group of the next AA. Peptide bond - Contains an N (free H3N+) terminal written on the left. Peptide bond - Contains a C (free COO-_ terminal written on the right. -ayl ending - the N- terminal the full AA name - The free carboxyl group at the C-terminal end. The primary structure of a protein - The particular sequence of AAs. The primary structure of a protein - The backbone of a peptide chain or protein. oxytocin and vasopressin - have similar primary structure oxytocin and vasopressin - Differ only in the AAs at positions 3 and 8. Insulin - The 1st protein to have its primary structure determined. Insulin - Has a primary structure of 2 polypeptides chains linked by disulfide bonds
Written for
- Institution
- Proteins, Enzymes, and Nucleic Acids
- Course
- Proteins, Enzymes, and Nucleic Acids
Document information
- Uploaded on
- August 7, 2023
- Number of pages
- 15
- Written in
- 2023/2024
- Type
- Summary
Subjects
-
proteins
-
enzymes
-
and nucleic acids
Also available in package deal