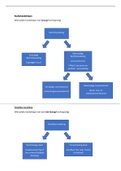
What is the basic structure of a nucleotide?
✔️✔️ A phosphate group, a sugar, and a nitrogenous base.
What is the primary role of chloroplasts in plant cells?
✔️✔️ To carry out photosynthesis.
What are the two purine bases in nucleic acids?
✔️✔️ Adenine and guanine.
What is the main function of the Golgi apparatus in a cell?
✔️✔️ To modify, package, and transport proteins and lipids.
What molecule acts as the final electron acceptor in the electron transport chain?
✔️✔️ Oxygen.
What are the end products of the citric acid cycle?
✔️✔️ CO₂, NADH, FADH₂, and ATP (or GTP).
What is the difference between competitive and non-competitive enzyme inhibition?
✔️✔️ Competitive inhibition blocks the active site, while non-competitive inhibition alters the
enzyme's shape by binding to a different site.
What is the structural feature that makes phospholipids amphipathic?
✔️✔️ They have a hydrophilic head and hydrophobic tails.
What process breaks down fatty acids into acetyl-CoA?
, ✔️✔️ Beta-oxidation.
What is the role of tRNA during protein synthesis?
✔️✔️ To carry specific amino acids to the ribosome and match them with the corresponding codons
on mRNA.
What is the function of lysosomes in a cell?
✔️✔️ To digest and recycle cellular waste and foreign materials.
What is the main difference between passive and active transport across a cell membrane?
✔️✔️ Passive transport does not require energy, while active transport requires ATP.
What is the role of coenzymes in enzymatic reactions?
✔️✔️ To assist enzymes by carrying chemical groups or electrons.
What is the main source of energy for muscle contraction?
✔️✔️ ATP.
What are the two main products of the light-dependent reactions in photosynthesis?
✔️✔️ ATP and NADPH.
What is the function of the sodium-potassium pump in cells?
✔️✔️ To maintain the electrochemical gradient by pumping sodium out and potassium into the cell.
What are the two main components of the cytoskeleton?
✔️✔️ Microfilaments and microtubules.
What is the main difference between exergonic and endergonic reactions?