Chemistry Notes Topic 2 - Predicting Structures, Bonding and Properties
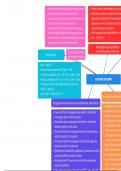
Using particles to determine physical properties:
● Melting/boiling points - determined by the strength of the intermolecular forces.
● Conductivity - only if it contains charged particles that are free to move.
● Solubility - depends on the type of particles that the substance contains.
Properties of different bonds:
● The properties of a material can be used to predict its structure, so it is very
important to know the main properties.
Using bonding to determine physical properties:
● You can also predict how a substance will behave depending on the type of bonding
it has.
● You need to consider the strong ionic, metallic or covalent bonds between atoms,
unless you’re dealing with simple covalent compounds, in which case you just need
to think about the intermolecular forces.
Using particles to determine physical properties:
● Melting/boiling points - determined by the strength of the intermolecular forces.
● Conductivity - only if it contains charged particles that are free to move.
● Solubility - depends on the type of particles that the substance contains.
Properties of different bonds:
● The properties of a material can be used to predict its structure, so it is very
important to know the main properties.
Using bonding to determine physical properties:
● You can also predict how a substance will behave depending on the type of bonding
it has.
● You need to consider the strong ionic, metallic or covalent bonds between atoms,
unless you’re dealing with simple covalent compounds, in which case you just need
to think about the intermolecular forces.