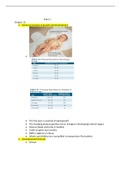
Density is the mass per unit volume (p=m/v)
Measure the mass of the object. Measure the volume of the container. Place the object in water
until is completely submerged. Measure the amount of water displaced. Subtract the initial amount
from final. Divide mass by volume to find the density.
Solids Liquid Gas
Very close together Close together Far apart
Regular pattern Random arrangement Random arrangement
Vibrate on the spot Move around each other Move quickly in all directions
Freezing- liquid to solid; The particles loose kinetic energy and begin to move slowly (ice cube).
Melting- solid to liquid; The particles gain kinetic energy and begin to move faster (chocolate).
Evaporation- liquid to gas; The particles gain kinetic energy and begin to move faster (sweating).
Condensation- gas to liquid; The particles loose kinetic energy and begin to move slowly (rain).
Deposition- gas to solid- The particles loose kinetic energy and begin to move slowly (water vapour).
Sublimation- solid to gas- The particles gain kinetic energy and begin to move faster (dry ice).
Evaporation happens at the surface and no bubbles are formed. The particles move and vibrate so
fast that they escape into the atmosphere. Boiling happens all through the liquid and it bubbles. The
particles are given more energy and move faster and faster expanding the liquid.
Internal energy is the total kinetic energy and potential energy of all the particles that make up a
system. Heating changes the energy stored within the system by increasing the energy of the
particles that make up a system This either increases the temperature or produces a change of state.
Internal energy is affected by temperature or pressure.
Specific Heat Capacity is how many joules of heat energy are needed to raise the temperature of:
each kg by each ℃ . (E= m*c*Θ )
Specific Latent Heat is the energy needed to change the state of 1kg of a substance without a change
in temperature. (E=m∠)
Fusion= melting (solid to liquid)
Vaporisation= boiling(liquid to gas)
Measure the mass of the object. Measure the volume of the container. Place the object in water
until is completely submerged. Measure the amount of water displaced. Subtract the initial amount
from final. Divide mass by volume to find the density.
Solids Liquid Gas
Very close together Close together Far apart
Regular pattern Random arrangement Random arrangement
Vibrate on the spot Move around each other Move quickly in all directions
Freezing- liquid to solid; The particles loose kinetic energy and begin to move slowly (ice cube).
Melting- solid to liquid; The particles gain kinetic energy and begin to move faster (chocolate).
Evaporation- liquid to gas; The particles gain kinetic energy and begin to move faster (sweating).
Condensation- gas to liquid; The particles loose kinetic energy and begin to move slowly (rain).
Deposition- gas to solid- The particles loose kinetic energy and begin to move slowly (water vapour).
Sublimation- solid to gas- The particles gain kinetic energy and begin to move faster (dry ice).
Evaporation happens at the surface and no bubbles are formed. The particles move and vibrate so
fast that they escape into the atmosphere. Boiling happens all through the liquid and it bubbles. The
particles are given more energy and move faster and faster expanding the liquid.
Internal energy is the total kinetic energy and potential energy of all the particles that make up a
system. Heating changes the energy stored within the system by increasing the energy of the
particles that make up a system This either increases the temperature or produces a change of state.
Internal energy is affected by temperature or pressure.
Specific Heat Capacity is how many joules of heat energy are needed to raise the temperature of:
each kg by each ℃ . (E= m*c*Θ )
Specific Latent Heat is the energy needed to change the state of 1kg of a substance without a change
in temperature. (E=m∠)
Fusion= melting (solid to liquid)
Vaporisation= boiling(liquid to gas)